MOUNTAIN VIEW, Calif., -- VIVUS, Inc. , today announced results of the company's Phase 2b clinical study of its investigational drug ALISTA(TM) (topical alprostadil), for the treatment of female sexual arousal disorder (FSAD), in women who have undergone a hysterectomy. In this double-blind, placebo-controlled study, patients with FSAD using ALISTA achieved a more than doubling over baseline in the number of satisfactory sexual events; however, the difference between the ALISTA treatment group and the placebo group did not achieve statistical significance for the primary endpoint of the study. Following a 2-month non-treatment run-in period, 320 subjects were randomly assigned to treatment with ALISTA or placebo for a period of 6 months. During study participation, subjects maintained daily diaries to capture outcomes of all sexual encounters, and the primary measure of treatment efficacy was based on the difference between ALISTA and placebo in the improvement over baseline in the number of satisfactory sexual encounters per month. This was the first study in which the efficacy of ALISTA was prospectively evaluated in an all organically impaired group of patients with FSAD.
"The placebo response rate was higher than what was anticipated in this phase 2b trial," said Leland Wilson, president and CEO of VIVUS. "Additional work will be required to determine how to better control the placebo response rate in this patient population before additional clinical trials can be started. Because we have a rich pipeline with three late-stage development programs, ALISTA will receive a lower development priority at VIVUS. We will focus our development efforts on Qnexa for the treatment of obesity, Testosterone MDTS for the treatment of hypoactive sexual desire disorder (HSDD) and avanafil for the treatment of male erectile dysfunction (MED), all of which have demonstrated clear, clinical and statistical significance over placebo in phase 2 studies."
I put this up for the simple reason that the people that received placebo may have even had a higher response. The mind is a very powerful thing! It reminds me of winter colds and people that drink tons of vitamin c, zinc etc. If you think that helps to make you feel better, it will. They have an interesting pipeline, Will keep an eye on this company.
Vivus trades on the NASDAQ: VVUS and was down at friday's close 23 cents to $3.70.
Saturday, September 30, 2006
Friday, September 29, 2006
Dow turns best third quarter in 11 years!
NEW YORK-- U.S. stocks closed lower on the day Friday, although the major averages logged solid quarterly gains due in large part to lower oil prices, allowing the Dow Jones Industrial Average to give its best third-quarter performance in 11 years.
A string of economic reports over the last three months also helped investors rally to the idea that U.S. economic growth will moderate, but remain at adequate levels to sustain corporate profits and job creation.
The Dow industrials ($INDU : Dow Jones Industrial Average News , chart, profile, more
Last: 11,679.07-39.38-0.34% closed down 39.38 points at 11,679.07.
On several occasions in Friday's the session, the benchmark index topped its record-high close of 11,722.98 from Jan. 14, 2000. The Dow's intraday record, set that same day, remains 11,750.28.
The Nasdaq Composite Index ($COMPQ : Nasdaq Composite Index fell 11.59 points to 2,258.43 while the S&P 500 Index ($SPX : S&P 500 Index
$SPX1,335.85, -3.30, -0.2%) lost 3.30 points to end at 1,335.85.
For the quarter the Dow gained 4.7%, its strongest advance since the third quarter of 1995.
The Nasdaq rose 4% in the quarter, making for its best third-quarter performance since 2003. The S&P 500 advanced 5.2% in the quarter.
"The gains we've seen derive from the conclusion that the economy is slowing, but not contracting or turning lower but there is not a high level of certainty in that conclusion. That's why the market is inching higher rather than striding higher," said Hugh Johnson, chairman of Johnson Illington Advisors.
Johnson said the quarter has been helped by a sharp fall in energy prices that has helped offset some of the concern over a slowing housing market and its impact on economic growth.
At the end of the third quarter, General Motors Corp. (GM : General Motors Corporation remains the top performer on the Dow since the beginning of 2006, with a staggering 70% gain. It places the car maker well above the No. 2 and 3 gainers, drug company Merck & Co. both of which are up about 32%.
A string of economic reports over the last three months also helped investors rally to the idea that U.S. economic growth will moderate, but remain at adequate levels to sustain corporate profits and job creation.
The Dow industrials ($INDU : Dow Jones Industrial Average News , chart, profile, more
Last: 11,679.07-39.38-0.34% closed down 39.38 points at 11,679.07.
On several occasions in Friday's the session, the benchmark index topped its record-high close of 11,722.98 from Jan. 14, 2000. The Dow's intraday record, set that same day, remains 11,750.28.
The Nasdaq Composite Index ($COMPQ : Nasdaq Composite Index fell 11.59 points to 2,258.43 while the S&P 500 Index ($SPX : S&P 500 Index
$SPX1,335.85, -3.30, -0.2%) lost 3.30 points to end at 1,335.85.
For the quarter the Dow gained 4.7%, its strongest advance since the third quarter of 1995.
The Nasdaq rose 4% in the quarter, making for its best third-quarter performance since 2003. The S&P 500 advanced 5.2% in the quarter.
"The gains we've seen derive from the conclusion that the economy is slowing, but not contracting or turning lower but there is not a high level of certainty in that conclusion. That's why the market is inching higher rather than striding higher," said Hugh Johnson, chairman of Johnson Illington Advisors.
Johnson said the quarter has been helped by a sharp fall in energy prices that has helped offset some of the concern over a slowing housing market and its impact on economic growth.
At the end of the third quarter, General Motors Corp. (GM : General Motors Corporation remains the top performer on the Dow since the beginning of 2006, with a staggering 70% gain. It places the car maker well above the No. 2 and 3 gainers, drug company Merck & Co. both of which are up about 32%.
Alvine Aims at Celiac Disease: Keeping an eye on this one.
Founded about a year ago to develop treatments for celiac disease, Alvine Pharmaceuticals Inc, recently raised $21 million in its Series A round to advance its lead product through proof of concept studies.
Celiac disease is an inherited autoimmune disease triggered by the consumption of foods containing gluten, a cereal grain protein. Considered fairly common, affecting as many of ine in 100 people in the US, it has historically been under-diagnosed with no available drug therapy. The only option for most celiac patients is to eliminate gluten from their diet, but that's not an easy feat given that it's found in so many foods, generally anything containing wheat or flour.
Alvine, which gets its name from a medical term referring to gastrointestinal activity, aims to develop a drug therapy that would allow celiac patients to consume "reasonable quantities" of gluten.
The company's lead product, ALV001, is a protease is expected to enter the clinic next year as a oral product, designed to be taken with food to break down and detoxify gluten. It accelerates the digestion of gluten before it arrives in the small intestine.
Celiac disease is an inherited autoimmune disease triggered by the consumption of foods containing gluten, a cereal grain protein. Considered fairly common, affecting as many of ine in 100 people in the US, it has historically been under-diagnosed with no available drug therapy. The only option for most celiac patients is to eliminate gluten from their diet, but that's not an easy feat given that it's found in so many foods, generally anything containing wheat or flour.
Alvine, which gets its name from a medical term referring to gastrointestinal activity, aims to develop a drug therapy that would allow celiac patients to consume "reasonable quantities" of gluten.
The company's lead product, ALV001, is a protease is expected to enter the clinic next year as a oral product, designed to be taken with food to break down and detoxify gluten. It accelerates the digestion of gluten before it arrives in the small intestine.
Corcept Therapeutics Inc. Announces Negative Results From Phase 3 Studies Evaluating CORLUX(R)
MENLO PARK, Calif., Sept. 29 -- Corcept Therapeutics Incorporated (Nasdaq: CORT - News), today announced that the second of its three Phase 3 trials evaluating CORLUX for treating the psychotic features of Psychotic Major Depression (PMD) was negative.
Study 09 was a randomized, double-blind, placebo-controlled study. The primary endpoint, a responder analysis, was the proportion of patients with at least a 50 percent improvement in the Brief Psychiatric Rating Scale Positive Symptom Subscale (BPRS PSS) at both Day 7 and Day 28. Specifically, the BPRS is an 18-item rating instrument used to assess psychopathology, and the PSS is a subset of four items in the BPRS that specifically measure psychosis. The study revealed no meaningful separation in response between patients receiving CORLUX and patients receiving placebo. The two key secondary endpoints of Study 09 were similarly negative.
"As was the case in Study 07, our previously announced Phase 3 clinical trial, there was an unusually high placebo response rate in Study 09," noted Robert L. Roe, M.D., Corcept's President and head of Development. "At Day 56, for example, approximately 95 percent of the patients in both of the arms of the study were responders as measured by a 50 percent improvement in BPRS PSS score."
"Although not the primary or a key secondary endpoint, it is interesting to note that there was a statistically significant separation between the CORLUX and placebo groups on an endpoint commonly used to measure the efficacy of antipsychotic and antidepressant medications, change from baseline to study end, in this case, Day 56," said Joseph K. Belanoff, M.D., Corcept's Chief Executive Officer. "However, because of the already high degree of response in the placebo group, it is difficult to determine how much additional clinical utility is conferred by this finding."
Corcept now has one Phase 3 study in progress. "We continue to enroll patients in Study 06 and expect to announce the results of this trial early next year," said Dr. Belanoff.
I will update more late, very busy today at my research bench. Monkey blood anyone?
CORT:NASDAQ is down 28% today trading at .95 cents.
Study 09 was a randomized, double-blind, placebo-controlled study. The primary endpoint, a responder analysis, was the proportion of patients with at least a 50 percent improvement in the Brief Psychiatric Rating Scale Positive Symptom Subscale (BPRS PSS) at both Day 7 and Day 28. Specifically, the BPRS is an 18-item rating instrument used to assess psychopathology, and the PSS is a subset of four items in the BPRS that specifically measure psychosis. The study revealed no meaningful separation in response between patients receiving CORLUX and patients receiving placebo. The two key secondary endpoints of Study 09 were similarly negative.
"As was the case in Study 07, our previously announced Phase 3 clinical trial, there was an unusually high placebo response rate in Study 09," noted Robert L. Roe, M.D., Corcept's President and head of Development. "At Day 56, for example, approximately 95 percent of the patients in both of the arms of the study were responders as measured by a 50 percent improvement in BPRS PSS score."
"Although not the primary or a key secondary endpoint, it is interesting to note that there was a statistically significant separation between the CORLUX and placebo groups on an endpoint commonly used to measure the efficacy of antipsychotic and antidepressant medications, change from baseline to study end, in this case, Day 56," said Joseph K. Belanoff, M.D., Corcept's Chief Executive Officer. "However, because of the already high degree of response in the placebo group, it is difficult to determine how much additional clinical utility is conferred by this finding."
Corcept now has one Phase 3 study in progress. "We continue to enroll patients in Study 06 and expect to announce the results of this trial early next year," said Dr. Belanoff.
I will update more late, very busy today at my research bench. Monkey blood anyone?
CORT:NASDAQ is down 28% today trading at .95 cents.
Thursday, September 28, 2006
Threshold Pharmaceuticals Gets Orphan Drug Status--Shares Rise
Biotech drugmaker Threshold Pharmaceuticals Inc. said Thursday the Food and Drug Administration granted orphan drug status to its treatment for pancreatic cancer.
In August, the company said it was focusing on a late-stage clinical trial of glufosfamide for the second-line treatment of pancreatic cancer and a mid-stage clinical trial, in combination with another drug, for an initial treatment.
Orphan drug status is given to drugs meant to treat conditions affecting fewer than 200,000 patients in the United States.
The announcement caused shares to jump. Shares of Threshold rose 35 cents, or 14 percent, to $2.85 in early morning trading on the Nasdaq.
The company's shares hit a 52-week high of $16.98 in April but plummeted in May when the FDA found a safety risk with the company's treatment for enlarged prostate. They dropped again in July when the company discontinued development of the drug.
The orphan status allows a company seven years of market exclusivity for the particular use if approved, along with tax breaks, development funding, and other incentives.
WHAT IS GLUFOSFAMIDE? Glufosfamide Is a next-generation glucose conjugate of ifosfamide. It is an alkylating agent [Binds to DNA stops replication] in which isophosphoramide mustard, the alkylating metabolite of ifosfamide, is glycosidically linked to beta-D-glucose. Cellular uptake of glufosfamide is mediated by a sodium-dependent transmembrane transporter protein of glucose and possibly also by other transporter proteins. They are exploiting unique aspects of tumour metabolism, particularly the elevated glucose utilization of tumour cells [Remember pancreas]to selectively target glufosfamide to the tumour site. Pretty cool science there. !
Currently Threshold (THLD) is trading on the NASDAQ up 14% to 2.87.
In August, the company said it was focusing on a late-stage clinical trial of glufosfamide for the second-line treatment of pancreatic cancer and a mid-stage clinical trial, in combination with another drug, for an initial treatment.
Orphan drug status is given to drugs meant to treat conditions affecting fewer than 200,000 patients in the United States.
The announcement caused shares to jump. Shares of Threshold rose 35 cents, or 14 percent, to $2.85 in early morning trading on the Nasdaq.
The company's shares hit a 52-week high of $16.98 in April but plummeted in May when the FDA found a safety risk with the company's treatment for enlarged prostate. They dropped again in July when the company discontinued development of the drug.
The orphan status allows a company seven years of market exclusivity for the particular use if approved, along with tax breaks, development funding, and other incentives.
WHAT IS GLUFOSFAMIDE? Glufosfamide Is a next-generation glucose conjugate of ifosfamide. It is an alkylating agent [Binds to DNA stops replication] in which isophosphoramide mustard, the alkylating metabolite of ifosfamide, is glycosidically linked to beta-D-glucose. Cellular uptake of glufosfamide is mediated by a sodium-dependent transmembrane transporter protein of glucose and possibly also by other transporter proteins. They are exploiting unique aspects of tumour metabolism, particularly the elevated glucose utilization of tumour cells [Remember pancreas]to selectively target glufosfamide to the tumour site. Pretty cool science there. !
Currently Threshold (THLD) is trading on the NASDAQ up 14% to 2.87.
Tokyo and Sydney advance overnight
Asian markets were broadly higher Thursday, with Japan and Australia posting gains for a second day as investors piled into Honda Motor Corp., BHP Billiton Ltd. and energy-related shares after crude-oil prices rebounded.
Japan's Nikkei 225 Average ended the session 0.5% higher to 16,024.9. The Tokyo Stock Price Index, or Topix, was up 0.7% to 1,602.6.
Elsewhere around the region, Australia's S&P ASX/200 rose 0.4% to 5,115.2, as the mining sector gained for a second day following recent weakness in the commodity sector.
Shares listed in India traded flat. Singapore's Straits Times Index was up 0.1%. Indonesia's Jakarta Composite Index rose 0.3%. New Zealand's leading share index rose
0.4%.
In Taipei, the Weighted Index fell 0.9% to 6,885.1, to rank as the lead declining market. Malaysia's KLSE Composite was up 0.3%. South Korea's Kospi Index was up 0.8%, while the Shanghai Composite Index advanced 0.7%.
Hong Kong's Hang Seng Index gained 0.2% to 17,551.5. The China Enterprises Index, or Hong Kong-listed shares in mainland-incorporated companies, was up 0.6% at 7,113.8.
"The new cabinet has started and there are upbeat expectations of the growth strategy of the Abe administration," said Hirokaza Yuihama, regional head of strategy for Daiwa Securities in Hong Kong. He added that U.S. data on new-home sales released Wednesday was stronger than expected, pointing to a more upbeat outlook for economic growth than many had expected.
"I have been positive on Asia because of the high probability of a soft landing in the U.S," Yuihama said. "It is unlikely we'll see further interest rate hikes this year, and we're even looking at a rate cut early next year, so it all works toward the positive."
Gains among Asian markets were limited despite Wednesday's advance on Wall Street, which saw the Dow Jones Industrial Average within striking distance of its all-time high.
Japan's Nikkei 225 Average ended the session 0.5% higher to 16,024.9. The Tokyo Stock Price Index, or Topix, was up 0.7% to 1,602.6.
Elsewhere around the region, Australia's S&P ASX/200 rose 0.4% to 5,115.2, as the mining sector gained for a second day following recent weakness in the commodity sector.
Shares listed in India traded flat. Singapore's Straits Times Index was up 0.1%. Indonesia's Jakarta Composite Index rose 0.3%. New Zealand's leading share index rose
0.4%.
In Taipei, the Weighted Index fell 0.9% to 6,885.1, to rank as the lead declining market. Malaysia's KLSE Composite was up 0.3%. South Korea's Kospi Index was up 0.8%, while the Shanghai Composite Index advanced 0.7%.
Hong Kong's Hang Seng Index gained 0.2% to 17,551.5. The China Enterprises Index, or Hong Kong-listed shares in mainland-incorporated companies, was up 0.6% at 7,113.8.
"The new cabinet has started and there are upbeat expectations of the growth strategy of the Abe administration," said Hirokaza Yuihama, regional head of strategy for Daiwa Securities in Hong Kong. He added that U.S. data on new-home sales released Wednesday was stronger than expected, pointing to a more upbeat outlook for economic growth than many had expected.
"I have been positive on Asia because of the high probability of a soft landing in the U.S," Yuihama said. "It is unlikely we'll see further interest rate hikes this year, and we're even looking at a rate cut early next year, so it all works toward the positive."
Gains among Asian markets were limited despite Wednesday's advance on Wall Street, which saw the Dow Jones Industrial Average within striking distance of its all-time high.
Wednesday, September 27, 2006
Today's picture: Calcium Waves

Calcium waves are intracellular calcium ions that can travel from cell to cell to propagate a specific signal. It occurs in electrical conducting cells such as heart cells, neurons and astrocytes. But they can occur in just about any cell population. They travel from cell to cell via gap junctions, connexons, and other ion channels. The above is a calcium wave extending out like a rock thrown into water. They are VERY fast and short lived. The photo intervals in the above were less than half a second.
FDA Approves Amgen Vectibix to treat Metastatic Colorectal Cancer
The target of AMGN's Vectibix: The Epidermal Growth Factor Receptor:

THOUSAND OAKS, Calif.-Amgen today announced that the U.S. Food and Drug Administration (FDA) has approved Vectibix(TM) (panitumumab) [Remember when I said you could instantly find targeted antibodies by the name XXXX-mab? Here's another example] following priority review. Vectibix is the first entirely human monoclonal antibody for the treatment of patients with epidermal growth factor receptor- (EGFr) expressing metastatic colorectal cancer after disease progression on, or following fluoropyrimidine-, oxaliplatin-, and irinotecan- containing chemotherapy regimens, e.g., NOT TREATABLE.
The FDA approval of Vectibix was based on a progression-free survival endpoint [not getting any worse]. Vectibix is the first anti-EGFr antibody shown to significantly improve progression-free survival in patients with metastatic colorectal cancer. Currently no data are available that demonstrate an improvement in disease-related symptoms or increased survival with Vectibix. Vectibix can be conveniently administered intravenously once every two weeks.
Epidermal growth factor receptors are proteins that play an important role in cancer cell signaling.
Another anti-EGFR familty therapy is Herceptin, specific for the related family member Her-2 for breast cancer. EGFR is widely expressing in skin, the gut and Her-2 in the heart. Such being, patients have severe reeactions to the skin and intestinal tract. In fact, it's been my research experience that herceptin patients usually die of heart failure than breast cancer.
READ THE PRODUCT LABEL I FOUND:
Dermatologic toxicities, related to Vectibix blockade of EGF binding and subsequent inhibition of EGF receptor-mediated signaling pathways, included but were not limited to dermatitis acneiform, pruritus, erythema, rash, skin exfoliation, paronychia, dry skin, and skin fissures [ CRACKS OPENING UP]. Dermatologic toxicities were reported in 89 percent of patients treated with Vectibix and were severe in 12 percent of patients. Severe dermatologic toxicities were complicated by infection, including sepsis, septic death, and abscesses requiring incisions and drainage. Vectibix may need to be withheld or discontinued for severe dermatologic toxicities.
Amgen NASDAQ: AMGN finished up today about 2% to 72.14.

THOUSAND OAKS, Calif.-Amgen today announced that the U.S. Food and Drug Administration (FDA) has approved Vectibix(TM) (panitumumab) [Remember when I said you could instantly find targeted antibodies by the name XXXX-mab? Here's another example] following priority review. Vectibix is the first entirely human monoclonal antibody for the treatment of patients with epidermal growth factor receptor- (EGFr) expressing metastatic colorectal cancer after disease progression on, or following fluoropyrimidine-, oxaliplatin-, and irinotecan- containing chemotherapy regimens, e.g., NOT TREATABLE.
The FDA approval of Vectibix was based on a progression-free survival endpoint [not getting any worse]. Vectibix is the first anti-EGFr antibody shown to significantly improve progression-free survival in patients with metastatic colorectal cancer. Currently no data are available that demonstrate an improvement in disease-related symptoms or increased survival with Vectibix. Vectibix can be conveniently administered intravenously once every two weeks.
Epidermal growth factor receptors are proteins that play an important role in cancer cell signaling.
Another anti-EGFR familty therapy is Herceptin, specific for the related family member Her-2 for breast cancer. EGFR is widely expressing in skin, the gut and Her-2 in the heart. Such being, patients have severe reeactions to the skin and intestinal tract. In fact, it's been my research experience that herceptin patients usually die of heart failure than breast cancer.
READ THE PRODUCT LABEL I FOUND:
Dermatologic toxicities, related to Vectibix blockade of EGF binding and subsequent inhibition of EGF receptor-mediated signaling pathways, included but were not limited to dermatitis acneiform, pruritus, erythema, rash, skin exfoliation, paronychia, dry skin, and skin fissures [ CRACKS OPENING UP]. Dermatologic toxicities were reported in 89 percent of patients treated with Vectibix and were severe in 12 percent of patients. Severe dermatologic toxicities were complicated by infection, including sepsis, septic death, and abscesses requiring incisions and drainage. Vectibix may need to be withheld or discontinued for severe dermatologic toxicities.
Amgen NASDAQ: AMGN finished up today about 2% to 72.14.
BioCryst Shares Rise on Bird Flu Study: Positive Peramivir Results
Shares of drug developer BioCryst Pharmaceuticals Inc. jumped Wednesday after the company said it will present positive study data on its influenza treatment at a weekend conference.
The treatment candidate, called peramivir, is in preclinical trials. The company said research at the University of Texas funded by the National Institute of Allergy and Infectious Diseases showed the drug's effectiveness on avian flu in mice and ferrets.
The company said this is the first data to describe the activity of peramivir in an established animal model using the highly pathogenic H5N1 strain of avian flu.
More than 200,000 people are hospitalized each year in the United States because of flu complications, according to the Centers for Disease Control and Prevention. About 36,000 people die each year from the flu. According to the World Health Organization, 247 people worldwide have contracted the H5N1 avian flu and 144 have died.
SO what is peramivir? It is a highly selective inhibitor of influenza A and B virus neuraminidases [a surface protein of the virus; they differ from viral strain to strain thus the reason for some flu virions are resistant to treatment] and a potent inhibitor of influenza A and B virus replication in cell culture. It prevents viral replication.
The stock gained 90 cents, or 7.1 percent, to reach $12.16 in morning trading on the Nasdaq. Shares have traded between $8.20 and $23 over the last 52 weeks.
The treatment candidate, called peramivir, is in preclinical trials. The company said research at the University of Texas funded by the National Institute of Allergy and Infectious Diseases showed the drug's effectiveness on avian flu in mice and ferrets.
The company said this is the first data to describe the activity of peramivir in an established animal model using the highly pathogenic H5N1 strain of avian flu.
More than 200,000 people are hospitalized each year in the United States because of flu complications, according to the Centers for Disease Control and Prevention. About 36,000 people die each year from the flu. According to the World Health Organization, 247 people worldwide have contracted the H5N1 avian flu and 144 have died.
SO what is peramivir? It is a highly selective inhibitor of influenza A and B virus neuraminidases [a surface protein of the virus; they differ from viral strain to strain thus the reason for some flu virions are resistant to treatment] and a potent inhibitor of influenza A and B virus replication in cell culture. It prevents viral replication.
The stock gained 90 cents, or 7.1 percent, to reach $12.16 in morning trading on the Nasdaq. Shares have traded between $8.20 and $23 over the last 52 weeks.
Pro Pharma shooting up today 38%!
This was the company I posted a few days ago talking about their carbohydrate drug targeting to lectins. The stock is (as of right now) at 90 cents. I will update PRW later today.
Picture of the Day: Mitosis
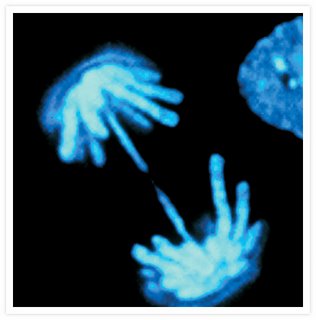
This is a specific stain for DNA (dapi) that when laser energy is introduced, it fluoresces blue. The strands you see are the individual chromosomes condensed and being separated from it's sister chromatid. Microtubules play a MAJOR role in mitosis, thus the interest in drugs to inhibit mitosis and microtubules.
Big Time Action!! Asian markets rally from US data
HONG KONG -- Japanese and Australian stocks led a broad advance in Asian markets through mid-session Wednesday, as investors snapped up consumer conglomerate Canon Inc. while BHP Billiton Ltd also attracted attention in the commodity complex after strong gains on Wall Street.
U.S. stock indexes hit fresh multi-year highs Tuesday after lower oil prices and data showing consumer confidence remained at higher levels than expected.
The Nikkei 225 Average (JP:1804610: news, chart, profile) ended the morning session up 1.7% to 15,821.2. The Topix index, which tracks about 1,600 companies on the first section of the Tokyo Stock Exchange, was up 1.9% to 1,579.3.
"Most of the reasons for the run are a reaction to the (Tuesday) sell-off and positive sentiment overnight in all the regional markets being stronger," said Miles Remington, head of equity trading at BNP Paribas in Hong Kong.
Remington said he was cautious on the short-term market outlook, but upbeat on a longer term view if a U.S. interest rate cut comes through early next year as expected.
U.S. stock indexes hit fresh multi-year highs Tuesday after lower oil prices and data showing consumer confidence remained at higher levels than expected.
The Nikkei 225 Average (JP:1804610: news, chart, profile) ended the morning session up 1.7% to 15,821.2. The Topix index, which tracks about 1,600 companies on the first section of the Tokyo Stock Exchange, was up 1.9% to 1,579.3.
"Most of the reasons for the run are a reaction to the (Tuesday) sell-off and positive sentiment overnight in all the regional markets being stronger," said Miles Remington, head of equity trading at BNP Paribas in Hong Kong.
Remington said he was cautious on the short-term market outlook, but upbeat on a longer term view if a U.S. interest rate cut comes through early next year as expected.
Tuesday, September 26, 2006
An Update from a Previous Post: Genentech Adds Brain Warning to Avastin Label
WASHINGTON - Genentech Inc. has added warnings about a rare brain condition called reversible posterior leukoencephalopathy syndrome, or RPLS, in patients using its cancer drug Avastin, the U.S. Food and Drug Administration said on Monday.
Remember that Avastin is the anti VEGF antibody. Stay tuned if anything else is posted in the science journals that isnt available to the public, it will be here. Genentech (NYSW: DNA) finished up today 1.4% at 79.66 in average trading.
Remember that Avastin is the anti VEGF antibody. Stay tuned if anything else is posted in the science journals that isnt available to the public, it will be here. Genentech (NYSW: DNA) finished up today 1.4% at 79.66 in average trading.
Millennium Pharma to acquire AnorMed---AnorMed shares Skyrocket
CAMBRIDGE-- Millennium Pharmaceuticals, Inc. today announced it has entered into an agreement to acquire AnorMED, Inc. , a Canadian-based biopharmaceutical company with a late-stage Phase III hematology-oncology product, MOZOBIL. Under the terms of the agreement approved by the boards of directors of both companies and the largest shareholder of AnorMED, Millennium will commence within ten days a cash tender offer to acquire the shares of AnorMED stock at a price of U.S. $12.00 per outstanding share, for a total purchase price of approximately $515 million. This represents approximately a 21 percent premium over the closing price of AnorMED's shares on September 25, 2006.
WHAT IS MOZOBIL AND HOW DOES IT WORK?
MOZOBIL is used in Cardiac Tissue Repair. Mozobil is a stem cell mobilizer. We all have heard of stem cells so that part is explained. This drug is telling the body to release it's own bone marrow derived stem cells to leave and enter the blood. It is a small molecule therapeutic that blocks a specific cellular receptor, CXCR4. CXCR4 is a cytokine [chemokine] receptor on many types of cells. By blocking this receptor it stops cancer cells from moving to other parts of the body. It does this by inhibiting cell homing briefly while the stem cells enter the blood. The stem cells can then be collected and used later in the patient.
Stem cells play an important role in the repair of damaged tissue and organs.
Anormed closed trading today up nearly 27% at 14.06 in heavy trading on the Toronto exchange. On the NASDAQ exchange (ANOR) closed up over 28% to 12.74. Millennium closed unchanged.
WHAT IS MOZOBIL AND HOW DOES IT WORK?
MOZOBIL is used in Cardiac Tissue Repair. Mozobil is a stem cell mobilizer. We all have heard of stem cells so that part is explained. This drug is telling the body to release it's own bone marrow derived stem cells to leave and enter the blood. It is a small molecule therapeutic that blocks a specific cellular receptor, CXCR4. CXCR4 is a cytokine [chemokine] receptor on many types of cells. By blocking this receptor it stops cancer cells from moving to other parts of the body. It does this by inhibiting cell homing briefly while the stem cells enter the blood. The stem cells can then be collected and used later in the patient.
Stem cells play an important role in the repair of damaged tissue and organs.
Anormed closed trading today up nearly 27% at 14.06 in heavy trading on the Toronto exchange. On the NASDAQ exchange (ANOR) closed up over 28% to 12.74. Millennium closed unchanged.
CytRX shares fall on Phase II arimoclomol results
CYTR shares fell 24% to $1.39 in Tuesday morning trade after the Los Angeles-based biopharmaceutical company reported results from its Phase IIa clinical trial for its lead drug candidate arimoclomol in patients with amyotrophic lateral sclerosis, or Lou Gehrig's disease. CytRx said arimoclomol was shown to be safe and well tolerated at all three doses tested, and the company plans to proceed with activities associated with initiating a Phase IIb clinical trial in the first half of 2007, subject to Food and Drug Administration approval. The company also said that "due to the limited size and duration of the trial" arimoclomol did not show a statistically significant change in disease progression as measured by certain markers.
Arimoclomol is a small molecule that’s designed to stimulate a natural cellular repair pathway by activating compounds called “molecular chaperones.” It has been shown to extend life in ALS-affected mice and was well tolerated in healthy human volunteers in a recently completed phase 1 study. Proteins that are not folded correctly help lead to the symptoms of ALS.
Another disease that is caused by misfolded proteins is Prion disease. If you have ever heard of mad cow, same thing but the human version is called Creutzfeldt-Jakob [in the brain].
The numbers have to show a significant effect on the disease or the trial is considered a failure.
The FDA has also granted arimoclomol “fast-track” status, a program begun in 1997 to hasten review of potential treatments for serious or life-threatening diseases.
CytRX shares closed trading today at $1.35
Arimoclomol is a small molecule that’s designed to stimulate a natural cellular repair pathway by activating compounds called “molecular chaperones.” It has been shown to extend life in ALS-affected mice and was well tolerated in healthy human volunteers in a recently completed phase 1 study. Proteins that are not folded correctly help lead to the symptoms of ALS.
Another disease that is caused by misfolded proteins is Prion disease. If you have ever heard of mad cow, same thing but the human version is called Creutzfeldt-Jakob [in the brain].
The numbers have to show a significant effect on the disease or the trial is considered a failure.
The FDA has also granted arimoclomol “fast-track” status, a program begun in 1997 to hasten review of potential treatments for serious or life-threatening diseases.
CytRX shares closed trading today at $1.35
Phase III data from Acorda sends stock soaring!
Shares of Acorda Therapeutics Inc, rocketed by more than 280% monday on positive findings from a phase III study of Fampridine-SR in multiple sclerosis.
The company's stock gained $6.28 to $8.50 because the drug enabled MS patients to walk with far less difficulty, a benefit that stands in contrast to current therapies, of which none are labeled for aiding in mobility.
The drug is expected to be used in a complementary role to existing MS drugs, with sales predicted to reach $300 million.
Fampridine SR is a sustained release oral drug that improves impulse conduction [electrical] in nerve fibers in which the insulating layer, called myelin, has been damaged. It is a potassium channel blocker [receptors in cells that regulate ion balance between in and out of cells] that helps nerve impulses conduct down axons.
Acorda NASDAQ:ACOR is up today as well 34% to $11.40 in very heavy trading.
The company's stock gained $6.28 to $8.50 because the drug enabled MS patients to walk with far less difficulty, a benefit that stands in contrast to current therapies, of which none are labeled for aiding in mobility.
The drug is expected to be used in a complementary role to existing MS drugs, with sales predicted to reach $300 million.
Fampridine SR is a sustained release oral drug that improves impulse conduction [electrical] in nerve fibers in which the insulating layer, called myelin, has been damaged. It is a potassium channel blocker [receptors in cells that regulate ion balance between in and out of cells] that helps nerve impulses conduct down axons.
Acorda NASDAQ:ACOR is up today as well 34% to $11.40 in very heavy trading.
Pro-Pharma updates product pipeline: It's the SUGARS!
Pro-Pharmaceuticals Updates Clinical Trials Progress
Pro-Pharmaceuticals, Inc.a developer of novel carbohydrate therapeutic compounds, today updated its clinical trials progress.
"We continue to make excellent progress in the clinic with DAVANAT®, our lead product candidate," said David Platt, Ph.D., Chief Executive Officer, Pro-Pharmaceuticals. "The outstanding results from our Phase I and Phase II trials of end-stage patients, who were refractory to chemotherapy, have led us to initiate three clinical trials with line one and line two patients. We believe we will improve the clinical benefit to patients over the current stand-of-care for colorectal and biliary cancer. We plan to begin dosing patients shortly.
"DAVANAT® is a complex carbohydrate drug that when given in combination with chemotherapeutic agents demonstrates reduced toxicity and increased efficacy by targeting the delivery and penetration of the chemotherapy to the tumor [see below targeting sugars to bind to sugars to target drugs]. The move to line l patients is recognition of the safety profile of DAVANAT®," Dr. Platt stated.
Clinical Trial Progress:
Phase I, line three/four, all Solid Tumors Trial and Phase II, line three/four, Colorectal Cancer Trial Results
Phase I and Phase II clinical trials of DAVANAT® with 5-FU data shows 1 patient with an objective partial tumor response and 20 patients stabilized out of 60. These patients had tumors, on average of 100mm,[that's big!] had a minimum of 12 weeks to live and were refractory to chemotherapy, including 5-FU. One patient from Phase II continues to be treated for 26 weeks. A Cholangiocarcinoma patient from our Phase I trial was treated for more than 56 weeks. The results of these studies compare very well and exceed results from similar recent studies in the same patient populations.
Phase II, line one, Colorectal Cancer Trial U.S.-based Phase II, line one clinical trial of DAVANAT® with
Avastin®, 5-FU and leucovorin for patients with locally advanced and unresectable [ can't be removed surgically] or metastatic colorectal cancer unable to tolerate intensive chemotherapy.
Phase III, line two, Colorectal Cancer Trial Europe-based Phase III, line two clinical trial for patients with
metastatic colorectal cancer is a multi-center, randomized with control groups study to evaluate the safety and efficacy of DAVANAT® in combination with 5-FU, leucovorin and irinotecan and/or oxaliplatin.
Phase II, line one, Biliary Cancer Trial U.S.-based Phase II, line one clinical trial of DAVANAT® with
5-FU [ 5 fluoro-uracil inhibits DNA synthesis, but is a very nasty chemical] for patients with biliary cancer. Biliary cancer may represent an opportunity for orphan drug status approval.
DAVANAT polysaccharide polymer [ a suger molecule] comprised of mannose and galactose carbohydrates in a CARBOSOME formation that enables the targeted delivery of chemotherapy drugs to protein receptors (lectins) [sugars binding to other sugars on the cell surface- a new way to target drugs to specific cells] on cancer cells.
Pro is down today on the AMEX (PRW) 13% to 0.64 cents.
Pro-Pharmaceuticals, Inc.a developer of novel carbohydrate therapeutic compounds, today updated its clinical trials progress.
"We continue to make excellent progress in the clinic with DAVANAT®, our lead product candidate," said David Platt, Ph.D., Chief Executive Officer, Pro-Pharmaceuticals. "The outstanding results from our Phase I and Phase II trials of end-stage patients, who were refractory to chemotherapy, have led us to initiate three clinical trials with line one and line two patients. We believe we will improve the clinical benefit to patients over the current stand-of-care for colorectal and biliary cancer. We plan to begin dosing patients shortly.
"DAVANAT® is a complex carbohydrate drug that when given in combination with chemotherapeutic agents demonstrates reduced toxicity and increased efficacy by targeting the delivery and penetration of the chemotherapy to the tumor [see below targeting sugars to bind to sugars to target drugs]. The move to line l patients is recognition of the safety profile of DAVANAT®," Dr. Platt stated.
Clinical Trial Progress:
Phase I, line three/four, all Solid Tumors Trial and Phase II, line three/four, Colorectal Cancer Trial Results
Phase I and Phase II clinical trials of DAVANAT® with 5-FU data shows 1 patient with an objective partial tumor response and 20 patients stabilized out of 60. These patients had tumors, on average of 100mm,[that's big!] had a minimum of 12 weeks to live and were refractory to chemotherapy, including 5-FU. One patient from Phase II continues to be treated for 26 weeks. A Cholangiocarcinoma patient from our Phase I trial was treated for more than 56 weeks. The results of these studies compare very well and exceed results from similar recent studies in the same patient populations.
Phase II, line one, Colorectal Cancer Trial U.S.-based Phase II, line one clinical trial of DAVANAT® with
Avastin®, 5-FU and leucovorin for patients with locally advanced and unresectable [ can't be removed surgically] or metastatic colorectal cancer unable to tolerate intensive chemotherapy.
Phase III, line two, Colorectal Cancer Trial Europe-based Phase III, line two clinical trial for patients with
metastatic colorectal cancer is a multi-center, randomized with control groups study to evaluate the safety and efficacy of DAVANAT® in combination with 5-FU, leucovorin and irinotecan and/or oxaliplatin.
Phase II, line one, Biliary Cancer Trial U.S.-based Phase II, line one clinical trial of DAVANAT® with
5-FU [ 5 fluoro-uracil inhibits DNA synthesis, but is a very nasty chemical] for patients with biliary cancer. Biliary cancer may represent an opportunity for orphan drug status approval.
DAVANAT polysaccharide polymer [ a suger molecule] comprised of mannose and galactose carbohydrates in a CARBOSOME formation that enables the targeted delivery of chemotherapy drugs to protein receptors (lectins) [sugars binding to other sugars on the cell surface- a new way to target drugs to specific cells] on cancer cells.
Pro is down today on the AMEX (PRW) 13% to 0.64 cents.
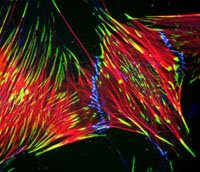
Tuesday word of the day: Focal Adhesion

This is a dual color image of a cell stained for integrins (see below) green and actin in red. The yellow color is where the two overlap.
Near and dear to my heart, the focal adhesion. The interface between the actin cytoskeleton and the extra cellular matrix, ECM, (plastic tissue culture dish or the neighboring cell--but called a CAM [cell adhesion molecule], is an important component of signal transduction of how the cell senses it's environment and how it reacts. If a cell does not attach to the ECM within around 15 minutes, it will apoptose. The places where it attaches to the surface of it's substrate, focal adhesion, has specific receptors termed integrins. The cell uses these interactions to determine it's environment and has a lot of signaling molecules around--[ see tyrosine phosphorylation from an earlier post] thus making this contact point a very important initiator of signaling.
A point to be made is that focal adhesions are an artifact of tissue culture. Cells are in a 3D dimension in the body and 2D in culture. I think i'm in the middle here. A ton of signal transduction/oncogene discovery has been accomplished, along with general cell biology from focal adhesion studies in vitro[ in tissue culture].
Monday, September 25, 2006
A Picture of Microtubules to see what the drug does....
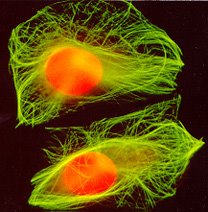
This is a picture of a cell stained with an antibody to microtubules. The cells were treated with taxol and the antibody was applied attaching directly to the stabilized microtubules (they are held in the polymerized position) and are visible by a fluorescent dye. The orange is a dye that stains the DNA, propidium iodide. I USED TO DO A LOT OF CYTOLOGY, THIS IS MY FAVORITE PICTURES TO TAKE!!!
EpiCept: Myriad triggers milestone payment for Azixa: Clinical Trials Look Promising
SAN FRANCISCO -- EpiCept Corp. Monday evening said Myriad Genetics Inc. has reported positive clinical results for Azixa, a compound discovered by EpiCept and licensed to Myriad. Based on the results, Myriad intends to initiate Phase II clinical trials, triggering a milestone payment to EpiCept. According to EpiCept, Myriad has reported that Azixa in cancer that has metastasized to the brain has achieved its maximum tolerated dose in patients, and there was a measured reduction in tumor size in certain patients.
Remember that metastasized means that the cancer cells have left the primary tumor site and relocated to another part of the body. The Phase I results mean that the drug is not toxic and has favorable pharmacokinetics.
Azixa(MPC-6827) is a novel small molecule inhibitor of microtubule formation that has demonstrated the ability to inhibit tumor growth in nonclinical testing, [in vitro and animals] with activity in animal models of human melanoma and cancers of the ovary, breast, prostate, colon and pancreas. In nonclinical testing, Azixa has been equally active against multiple drug resistant cancers as with susceptible tumors, unlike many of the current options in cancer chemotherapy, a limitation that represents a significant unmet medical need.
Among others, microtubule inhibitor drugs have been around for a long time. The best example of this class of drugs is taxol. Cells need to use microtubules like the bones in your body; they are the bones, along with actin, of the cell. The cancer cell divides a lot, so it has to continually depolymerize [break down] and then remake [polymerize] the microtubules. If the microtubules are forced to stay polymerized, the cell will under-go apoptosis (you should be reading...an earlier word of the day was apoptosis....cells committing suicide)
The most interesting thing about this drug is that it activates caspaces ( see earlier posts) selectively to help kill cancer cells. How it is targeted to specific tumor cells for caspaces remains to be seen.
Myriad closed up today 19 cents at 23.73 in about average trading. Epicept closed down 7 cents to 1.70 in robust trading. As a personal thought, I'm thinking of getting some action on Epicept. If these deals continue, may be up by the end of the year.
Remember that metastasized means that the cancer cells have left the primary tumor site and relocated to another part of the body. The Phase I results mean that the drug is not toxic and has favorable pharmacokinetics.
Azixa(MPC-6827) is a novel small molecule inhibitor of microtubule formation that has demonstrated the ability to inhibit tumor growth in nonclinical testing, [in vitro and animals] with activity in animal models of human melanoma and cancers of the ovary, breast, prostate, colon and pancreas. In nonclinical testing, Azixa has been equally active against multiple drug resistant cancers as with susceptible tumors, unlike many of the current options in cancer chemotherapy, a limitation that represents a significant unmet medical need.
Among others, microtubule inhibitor drugs have been around for a long time. The best example of this class of drugs is taxol. Cells need to use microtubules like the bones in your body; they are the bones, along with actin, of the cell. The cancer cell divides a lot, so it has to continually depolymerize [break down] and then remake [polymerize] the microtubules. If the microtubules are forced to stay polymerized, the cell will under-go apoptosis (you should be reading...an earlier word of the day was apoptosis....cells committing suicide)
The most interesting thing about this drug is that it activates caspaces ( see earlier posts) selectively to help kill cancer cells. How it is targeted to specific tumor cells for caspaces remains to be seen.
Myriad closed up today 19 cents at 23.73 in about average trading. Epicept closed down 7 cents to 1.70 in robust trading. As a personal thought, I'm thinking of getting some action on Epicept. If these deals continue, may be up by the end of the year.
Pharmion, GPC Biotech Shares Soar on Positive late-stage Satraplatin Study
NEW YORK-- Shares of several partnered biotechnology companies surged Monday after a prospective prostate cancer treatment lowered progression rates in a late-stage study.
Pharmion Corp. gained $3.92, or 21.8 percent, to reach $21.93 in afternoon trading on the Nasdaq, as trading volume rose more than sixfold from its average. Shares have traded between $14.76 and $23.34 over the last 52 weeks.
Meanwhile, American depositary shares of Germany-based GPC Biotech AG jumped $4.46, or 31.8 percent, to $18.46 on the Nasdaq, as trading volume surged to more than elevenfold. Early in the day, the stock climbed to a new 52-week high of $19.45, replacing the previous high of $18.59 set on Feb. 27. WOW!
The study showed that prostate cancer patients receiving satraplatin with prednisone had a 40 percent lower risk of disease progression, compared with those given placebo with prednisone. Boulder, Colo.-based Pharmion said it plans on filing a marketing application for the drug in Europe during the first half of 2007 while GPC Biotech plans to file with the Food and Drug Administration by the end of the year.
The news prompted Lazard Capital Markets analyst Matthew S. Osborne to upgrade Pharmion's stock to "Buy" from "Hold" with a $28 price target.
The assessment, which factors in competition, said the modest penetration of the European market could generate sales of $112 million by 2011 for the drug's current indication as a second-line treatment. Possible expansion to other uses leaves room for potential upside.
The stock rose $1.32, or 37.8 percent, to $4.81, as trading volume soared more than 32 times its average. Shares have traded between $3.36 and $5.69 over the last 52 weeks
What is satraplatin? It's a chemotherapy drug related to cisplatin. It is a DNA alkylating agent (binds and connects DNA to itself) that binds non specifically to cellular DNA and inhibits the cell from dividing. It has major antitumor activity in the genitourinary cancers particularly testicular, ovarian and bladder cancer. Inhibiting the cell from dividing causes the tumor to stop growing, eventually leading to the death of the cancer cells.
Side effects include kidney dysfunction, vomiting and hearing impairment. Not a delight.
Pharmion Corp. gained $3.92, or 21.8 percent, to reach $21.93 in afternoon trading on the Nasdaq, as trading volume rose more than sixfold from its average. Shares have traded between $14.76 and $23.34 over the last 52 weeks.
Meanwhile, American depositary shares of Germany-based GPC Biotech AG jumped $4.46, or 31.8 percent, to $18.46 on the Nasdaq, as trading volume surged to more than elevenfold. Early in the day, the stock climbed to a new 52-week high of $19.45, replacing the previous high of $18.59 set on Feb. 27. WOW!
The study showed that prostate cancer patients receiving satraplatin with prednisone had a 40 percent lower risk of disease progression, compared with those given placebo with prednisone. Boulder, Colo.-based Pharmion said it plans on filing a marketing application for the drug in Europe during the first half of 2007 while GPC Biotech plans to file with the Food and Drug Administration by the end of the year.
The news prompted Lazard Capital Markets analyst Matthew S. Osborne to upgrade Pharmion's stock to "Buy" from "Hold" with a $28 price target.
The assessment, which factors in competition, said the modest penetration of the European market could generate sales of $112 million by 2011 for the drug's current indication as a second-line treatment. Possible expansion to other uses leaves room for potential upside.
The stock rose $1.32, or 37.8 percent, to $4.81, as trading volume soared more than 32 times its average. Shares have traded between $3.36 and $5.69 over the last 52 weeks
What is satraplatin? It's a chemotherapy drug related to cisplatin. It is a DNA alkylating agent (binds and connects DNA to itself) that binds non specifically to cellular DNA and inhibits the cell from dividing. It has major antitumor activity in the genitourinary cancers particularly testicular, ovarian and bladder cancer. Inhibiting the cell from dividing causes the tumor to stop growing, eventually leading to the death of the cancer cells.
Side effects include kidney dysfunction, vomiting and hearing impairment. Not a delight.
Boston Life Sciences, Inc. Announces Findings from Review of POET-1 Study of ALTROPANE(R) Molecular Imaging Agent
Boston Life Sciences, Inc. today reported statistically significant results for the Primary Endpoint from the POET-1 (Parkinson's or Essential Tremor) trial for the ALTROPANE molecular imaging agent. The POET-1 trial was designed to assess whether ALTROPANE imaging is more accurate than the clinical diagnosis of primary care physicians (PCP) to distinguish between tremors caused by Parkinsonian Syndrome and those associated with other disorders, as judged by comparison to a definitive diagnosis by Movement Disorder Specialists (MDS). ALTROPANE scans showed statistically significant superiority over the diagnosis of PCPs on measures of both specificity and sensitivity, the Primary Endpoint of the trial. Based on data analyzed to date, with the exception of one "possibly-related" urinary tract infection that resolved after treatment, there were no drug-related serious adverse events. In order to avoid potential bias to further clinical development of ALTROPANE and in keeping with FDA guidance to the Company, detailed results of the trial cannot be disclosed at this time.
As previously announced in March 2006, the Company ended the POET-1 trial early with approximately 30 percent fewer patients than originally specified because non-blinded data indicated that the error rate of general practitioners in the trial was higher than had been anticipated in the trial design.
BLSI's President and Chief Operating Officer, Dr. Mark Pykett, said, "We believe these results will be instrumental in advancing our efforts to sign a commercial partner for ALTROPANE -- a primary business objective for our Molecular Imaging program. We are working with expert advisors and the FDA to determine the most-direct route to FDA approval, and to finalize our plans for POET-2 and the remaining clinical development of ALTROPANE. We plan to provide further guidance to our investors as we gain insight into what further work will be required to seek regulatory approval, including specifics regarding POET-2 and any other studies that may be necessary."
BLSI's Chief Medical Officer, Mark Hurtt, MD, commented, "This is the outcome we anticipated when we ended the POET-1 trial. Our results are particularly encouraging because the readers made their diagnoses based on their evaluations of the ALTROPANE scans alone; they had no access to patient clinical data, such as the age of the patient, in these "fully-blinded" evaluations. In normal practice, nuclear-medicine physicians would evaluate clinical information along with the scans, to further enhance their diagnostic accuracy."
Dr. Alan Waxman of Cedars Sinai Medical Center, a nuclear-medicine expert, a professor at the University of Southern California, and a principal investigator in the POET-1 trial, stated, "We found in our clinic that ALTROPANE was convenient to use, and routinely produced high-quality scans. We were able to complete each patient's testing in about an hour."
ALTROPANE is a molecular imaging agent that specifically binds to the dopamine transporter (DAT) protein found on the surface of dopamine-producing neurons, making it visible during Single Photon Emission Computed Tomography or SPECT, imaging. Since most forms of Parkinsonian Syndromes result in a decreased number of dopamine-producing cells, it would be expected that these patients also have fewer DATs than do patients without PS. Thus, we believe that ALTROPANE used in conjunction with SPECT imaging could be a useful test to distinguish Parkinsonian Syndrome tremors from non-Parkinsonian tremor: non-Parkinsonian patients would have more ALTROPANE-binding visible in the SPECT image, while Parkinsonian patients would have less.
Boston Life Sciences is traded on the Nasdaq index under the symbol BLSI. BLSI is in active trading today up 1.6% to 3.93 in afternoon trading.
As previously announced in March 2006, the Company ended the POET-1 trial early with approximately 30 percent fewer patients than originally specified because non-blinded data indicated that the error rate of general practitioners in the trial was higher than had been anticipated in the trial design.
BLSI's President and Chief Operating Officer, Dr. Mark Pykett, said, "We believe these results will be instrumental in advancing our efforts to sign a commercial partner for ALTROPANE -- a primary business objective for our Molecular Imaging program. We are working with expert advisors and the FDA to determine the most-direct route to FDA approval, and to finalize our plans for POET-2 and the remaining clinical development of ALTROPANE. We plan to provide further guidance to our investors as we gain insight into what further work will be required to seek regulatory approval, including specifics regarding POET-2 and any other studies that may be necessary."
BLSI's Chief Medical Officer, Mark Hurtt, MD, commented, "This is the outcome we anticipated when we ended the POET-1 trial. Our results are particularly encouraging because the readers made their diagnoses based on their evaluations of the ALTROPANE scans alone; they had no access to patient clinical data, such as the age of the patient, in these "fully-blinded" evaluations. In normal practice, nuclear-medicine physicians would evaluate clinical information along with the scans, to further enhance their diagnostic accuracy."
Dr. Alan Waxman of Cedars Sinai Medical Center, a nuclear-medicine expert, a professor at the University of Southern California, and a principal investigator in the POET-1 trial, stated, "We found in our clinic that ALTROPANE was convenient to use, and routinely produced high-quality scans. We were able to complete each patient's testing in about an hour."
ALTROPANE is a molecular imaging agent that specifically binds to the dopamine transporter (DAT) protein found on the surface of dopamine-producing neurons, making it visible during Single Photon Emission Computed Tomography or SPECT, imaging. Since most forms of Parkinsonian Syndromes result in a decreased number of dopamine-producing cells, it would be expected that these patients also have fewer DATs than do patients without PS. Thus, we believe that ALTROPANE used in conjunction with SPECT imaging could be a useful test to distinguish Parkinsonian Syndrome tremors from non-Parkinsonian tremor: non-Parkinsonian patients would have more ALTROPANE-binding visible in the SPECT image, while Parkinsonian patients would have less.
Boston Life Sciences is traded on the Nasdaq index under the symbol BLSI. BLSI is in active trading today up 1.6% to 3.93 in afternoon trading.
Gardasil is made to the capsid proteins of HPV
Gardasil is a non-infectious quadrivalent recombinant vaccine, which delivers the major capsid (L1) protein of human papillomavirus (HPV) types 6, 11, 16 and 18 in highly purified virus-like particles, in combination wth an aluminum-containing vaccine adjuvant.
Gardasil is specifically indicated for the prevention of conditions caused by HPV types 6, 11, 16 and 18 infections. These include cervical cancer, genital warts (condyloma acuminata), and precancerous or dysplastic lesions (including cervical adenocarcinoma in situ (AIS), cervical intraepithelial neoplasia (CIN) grades 1 and 2/3, vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3, and vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3).
Gardasil is supplies as a white, cloudy liquid for intramuscular injection. The recommended dosing regimen is 3 single injections of 0.5 ml of the vaccine, at day 0, 2 months after the first dose, and 6 months after the first dose.
Gardasil is a non-infectious quadrivalent recombinant vaccine, which delivers the major capsid (L1) protein of human papillomavirus (HPV) types 6, 11, 16 and 18 in highly purified virus-like particles, [the epitope]in combination wth an aluminum-containing vaccine adjuvant.
Gardasil is specifically indicated for the prevention of conditions caused by HPV types 6, 11, 16 and 18 infections. These include cervical cancer, genital warts (condyloma acuminata), and precancerous or dysplastic lesions (including cervical adenocarcinoma in situ (AIS), cervical intraepithelial neoplasia (CIN) grades 1 and 2/3, vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3, and vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3).
Gardasil is supplies as a white, cloudy liquid for intramuscular injection. The recommended dosing regimen is 3 single injections of 0.5 ml of the vaccine, at day 0, 2 months after the first dose, and 6 months after the first dose.
The capsid is the outer membrane of the virus.
Gardasil is specifically indicated for the prevention of conditions caused by HPV types 6, 11, 16 and 18 infections. These include cervical cancer, genital warts (condyloma acuminata), and precancerous or dysplastic lesions (including cervical adenocarcinoma in situ (AIS), cervical intraepithelial neoplasia (CIN) grades 1 and 2/3, vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3, and vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3).
Gardasil is supplies as a white, cloudy liquid for intramuscular injection. The recommended dosing regimen is 3 single injections of 0.5 ml of the vaccine, at day 0, 2 months after the first dose, and 6 months after the first dose.
Gardasil is a non-infectious quadrivalent recombinant vaccine, which delivers the major capsid (L1) protein of human papillomavirus (HPV) types 6, 11, 16 and 18 in highly purified virus-like particles, [the epitope]in combination wth an aluminum-containing vaccine adjuvant.
Gardasil is specifically indicated for the prevention of conditions caused by HPV types 6, 11, 16 and 18 infections. These include cervical cancer, genital warts (condyloma acuminata), and precancerous or dysplastic lesions (including cervical adenocarcinoma in situ (AIS), cervical intraepithelial neoplasia (CIN) grades 1 and 2/3, vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3, and vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3).
Gardasil is supplies as a white, cloudy liquid for intramuscular injection. The recommended dosing regimen is 3 single injections of 0.5 ml of the vaccine, at day 0, 2 months after the first dose, and 6 months after the first dose.
The capsid is the outer membrane of the virus.
Saturday, September 23, 2006
Busy Day with my own research today...
I will post some data I found last night as will as the specifics on Gardasil later today.
Friday, September 22, 2006
How Gardasil works post coming soon... I think it's worth a look. Cool Science
I'm reading and interpreting the data now.
Merck obtains License to Market Gardasil in Europe
WHITEHOUSE STATION, N.J.----GARDASIL(R) (Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine), the cervical cancer vaccine from Merck & Co., Inc., has been granted a license by the European Commission. GARDASIL has been approved as the first and only vaccine in the European Union (EU) for use in children and adolescents aged 9 to 15 years and in adult females aged 16 to 26 years for the prevention of cervical cancer, high-grade cervical dysplasias/precancers (cervical intraepithelial neoplasia [a different area of the cervix], high-grade/precancerous vulvar dysplastic lesions [precancer of the external vagina] and external genital warts caused by human papillomavirus (HPV) types 6, 11, 16 and 18. This license applies to the 25 countries that are members of the EU, including the five largest which are France, Germany, Italy, Spain and the United Kingdom.
Merck (MRK)closed up 9 cents today to finish at 42.04.
Merck (MRK)closed up 9 cents today to finish at 42.04.
EPIX depressed about phase III trial failure. Shares Plunge
EPIX Pharmaceuticals (EPIX) Announces Top-Line Results From Phase 3 Trial Of PRX-00023 In Generalized Anxiety Disorder; Drug Fails Trial
LEXINGTON, Mass.--Sept. 21, 2006--EPIX Pharmaceuticals, Inc. today announced that the top-line results from its Phase 3 trial of PRX-00023, a novel long-acting 5-HT1A agonist, show that overall PRX-00023 did not achieve a statistically significant improvement over placebo for the primary endpoint. The primary efficacy endpoint was change from baseline in Hamilton Rating Scale for Anxiety (HAM-A) compared to placebo. There was a trend in favor of patients treated with PRX-00023 in the HAM-A assessments; however, the outcome was potentially impacted by a higher than expected response in the placebo-treated patients.
The data from this trial did show a statistically significant improvement from baseline in the Montgomery Asberg Depression Rating Scale (MADRS) compared to placebo. The MADRS, which measures symptoms of depression, was a secondary endpoint in this trial. EPIX is continuing to analyze the data and will use these insights to guide its strategic planning process for the development of PRX-00023.
"We are disappointed that treatment with PRX-00023 did not induce a statistically significant change in the HAM-A in patients with anxiety; however, we are encouraged by the MADRS data and will further analyze these results to better understand the potential benefits of PRX-00023 in patients with depression," said Michael G. Kauffman, M.D., Ph.D., chief executive officer, EPIX Pharmaceuticals. "Based on these top-line results, we plan to refocus our efforts away from anxiety to evaluate the benefit in depression more closely and assess opportunities for initiating a Phase 2 clinical trial in depression sooner than originally planned."
A preliminary review has indicated that PRX-00023 was well tolerated and there was a low rate of discontinuation in this study due to adverse events. Side effects, including impact on sexual function and sleep, in patients receiving PRX-00023, were similar to placebo.
What is 5-HT? What is a 5-HT agonist? What's an agonist? How does this failed drug work and why did it fail?
Let's work through this. 5-HT is short for 5-hydroxytryptamine commonly called serotonin. It was identified by accident, when blood was allowed to clot a "tonic" substance was released that caused blood vessel constriction. Serotonin plays a role several diseases. The receptor for serotonin is 5-HT1a (for this drug) is located in the hippocampus of the brain and acts as a neurotransmitter.
An agonist binds to a particular receptor and activates it. This drug binds to the serotonin receptor and is supposed to block the cell signaling that leads to depression symptoms. It failed because the patients did not show a significant difference between the drug and placebo. It's interesting that two tests were used and it passed one.
These compounds are used as antidepressants, anxiolytics, and in the treatment of migraine.
Shares of EPIX plunged today 18% to $4.32
LEXINGTON, Mass.--Sept. 21, 2006--EPIX Pharmaceuticals, Inc. today announced that the top-line results from its Phase 3 trial of PRX-00023, a novel long-acting 5-HT1A agonist, show that overall PRX-00023 did not achieve a statistically significant improvement over placebo for the primary endpoint. The primary efficacy endpoint was change from baseline in Hamilton Rating Scale for Anxiety (HAM-A) compared to placebo. There was a trend in favor of patients treated with PRX-00023 in the HAM-A assessments; however, the outcome was potentially impacted by a higher than expected response in the placebo-treated patients.
The data from this trial did show a statistically significant improvement from baseline in the Montgomery Asberg Depression Rating Scale (MADRS) compared to placebo. The MADRS, which measures symptoms of depression, was a secondary endpoint in this trial. EPIX is continuing to analyze the data and will use these insights to guide its strategic planning process for the development of PRX-00023.
"We are disappointed that treatment with PRX-00023 did not induce a statistically significant change in the HAM-A in patients with anxiety; however, we are encouraged by the MADRS data and will further analyze these results to better understand the potential benefits of PRX-00023 in patients with depression," said Michael G. Kauffman, M.D., Ph.D., chief executive officer, EPIX Pharmaceuticals. "Based on these top-line results, we plan to refocus our efforts away from anxiety to evaluate the benefit in depression more closely and assess opportunities for initiating a Phase 2 clinical trial in depression sooner than originally planned."
A preliminary review has indicated that PRX-00023 was well tolerated and there was a low rate of discontinuation in this study due to adverse events. Side effects, including impact on sexual function and sleep, in patients receiving PRX-00023, were similar to placebo.
What is 5-HT? What is a 5-HT agonist? What's an agonist? How does this failed drug work and why did it fail?
Let's work through this. 5-HT is short for 5-hydroxytryptamine commonly called serotonin. It was identified by accident, when blood was allowed to clot a "tonic" substance was released that caused blood vessel constriction. Serotonin plays a role several diseases. The receptor for serotonin is 5-HT1a (for this drug) is located in the hippocampus of the brain and acts as a neurotransmitter.
An agonist binds to a particular receptor and activates it. This drug binds to the serotonin receptor and is supposed to block the cell signaling that leads to depression symptoms. It failed because the patients did not show a significant difference between the drug and placebo. It's interesting that two tests were used and it passed one.
These compounds are used as antidepressants, anxiolytics, and in the treatment of migraine.
Shares of EPIX plunged today 18% to $4.32
Word of the day, Friday: Neopterin
Neopterin is a chemical released by macrophages in response to interferon-gamma. It is a common marker of immune system activation, mostly used in monitoring HIV patients. Immune modulation by T cells releasing IFN-g, IFN-alpha (cytokines) is a good indication of immune activation.
Alcon, Inc.'s TRAVATAN(R) Z Solution Approved By FDA For Treatment Of Glaucoma And Ocular Hypertensive Patients
Alcon, Inc.'s TRAVATAN(R) Z Solution Approved By FDA For Treatment Of Glaucoma And Ocular Hypertensive Patients
Sept. 21, 2006--Alcon, Inc. (NYSE:ACL - News) announced today that the U.S. Food and Drug Administration (FDA) has approved TRAVATAN® Z (travoprost ophthalmic solution) 0.004% for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension, who are intolerant of or insufficiently responsive to other intraocular pressure lowering medications. TRAVATAN® Z is a new formulation that eliminates benzalkonium chloride (BAK) from Alcon's existing TRAVATAN® solution and replaces BAK with SOFZIA(TM), a robust ionic buffered preservative system that is gentle to the ocular surface. Alcon developed this BAK-free version of TRAVATAN® because long-term use of topical solutions containing BAK may compromise the ocular surface and exacerbate conditions such as dry eye.
"Because almost 40 percent of glaucoma patients suffer from Ocular Surface Disease, TRAVATAN® Z is an advance in therapy which we believe will now enable doctors to address an unmet need of many glaucoma patients," said Kevin Buehler, Alcon's senior vice president, United States and chief marketing officer.
BAK is an anti bacterial preservative that is in most IV bags and sterile solutions. I use it in the lab to preserve western blot antibody solutions that are diluted in milk. I also use it to sterilize my cell incubators
(NYSE:ACL-- Closed today down 1.71 at 114.29.
Sept. 21, 2006--Alcon, Inc. (NYSE:ACL - News) announced today that the U.S. Food and Drug Administration (FDA) has approved TRAVATAN® Z (travoprost ophthalmic solution) 0.004% for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension, who are intolerant of or insufficiently responsive to other intraocular pressure lowering medications. TRAVATAN® Z is a new formulation that eliminates benzalkonium chloride (BAK) from Alcon's existing TRAVATAN® solution and replaces BAK with SOFZIA(TM), a robust ionic buffered preservative system that is gentle to the ocular surface. Alcon developed this BAK-free version of TRAVATAN® because long-term use of topical solutions containing BAK may compromise the ocular surface and exacerbate conditions such as dry eye.
"Because almost 40 percent of glaucoma patients suffer from Ocular Surface Disease, TRAVATAN® Z is an advance in therapy which we believe will now enable doctors to address an unmet need of many glaucoma patients," said Kevin Buehler, Alcon's senior vice president, United States and chief marketing officer.
BAK is an anti bacterial preservative that is in most IV bags and sterile solutions. I use it in the lab to preserve western blot antibody solutions that are diluted in milk. I also use it to sterilize my cell incubators
(NYSE:ACL-- Closed today down 1.71 at 114.29.
Thursday, September 21, 2006
Medivation Shares Soar on Study Results
SAN FRANCISCO — Biotechnology company Medivation Inc. said Thursday a midstage study testing its Dimebon treatment for Alzheimer's showed the drug was significantly effective, sending shares soaring in morning trading.
The Phase II clinical trial involved 183 patients with mild to moderate Alzheimer's disease at 11 sites in Russia. The drug met all five of its efficacy endpoints, which included different measurement scales for improvement in the disease, during the six-month, randomized and double-blind study.
Results also showed the drug was well tolerated with mild side effects.
"We believe that these results are important, in part because the primary and key secondary efficacy endpoints used in this trial are accepted by the Food and Drug Administration for registration of drugs to treat mild to moderate Alzheimer's disease," said Dr. David Hung, Medivation president and chief executive, in a statement.
The company has also started an extension study on the drug, which allows patients to continue treatment for up to a total of 12 months. Those results are expected in the second quarter of 2007.
Dimebon is also being studied for possible use in treating Huntington's disease.
SO, what is Dimebon and how does it work?
Dimebon was tested in Russia as an antihistamine drug and was evaluated as a representative of a new generation of anti-Alzheimer's drugs that have two beneficial actions: (1) to alleviate symptoms, and (2) to prevent progression of the disease.
The drug demonstrated cognition and memory-enhancing properties in the active avoidance test in rats treated with the neurotoxin AF64A, which selectively destroys cholinergic neurons. Dimebon protected neurons in the cerebellum cell culture against the neurotoxic action of a test protein. In vitro, Dimebon displayed Ca2+-blocking properties and pronounced anticholinesterase activity. It also exhibited strong anti-NMDA ( an ion channel receptor) activity in the prevention of NMDA-induced seizures in mice.
It works by blocking L-type calcium channels that let in Calcium which destorys neurons.
Shares surged $1.75, or 29 percent, to $7.80 on the American Stock Exchange after setting a 52-week high of $8.10 earlier in the session.
The Phase II clinical trial involved 183 patients with mild to moderate Alzheimer's disease at 11 sites in Russia. The drug met all five of its efficacy endpoints, which included different measurement scales for improvement in the disease, during the six-month, randomized and double-blind study.
Results also showed the drug was well tolerated with mild side effects.
"We believe that these results are important, in part because the primary and key secondary efficacy endpoints used in this trial are accepted by the Food and Drug Administration for registration of drugs to treat mild to moderate Alzheimer's disease," said Dr. David Hung, Medivation president and chief executive, in a statement.
The company has also started an extension study on the drug, which allows patients to continue treatment for up to a total of 12 months. Those results are expected in the second quarter of 2007.
Dimebon is also being studied for possible use in treating Huntington's disease.
SO, what is Dimebon and how does it work?
Dimebon was tested in Russia as an antihistamine drug and was evaluated as a representative of a new generation of anti-Alzheimer's drugs that have two beneficial actions: (1) to alleviate symptoms, and (2) to prevent progression of the disease.
The drug demonstrated cognition and memory-enhancing properties in the active avoidance test in rats treated with the neurotoxin AF64A, which selectively destroys cholinergic neurons. Dimebon protected neurons in the cerebellum cell culture against the neurotoxic action of a test protein. In vitro, Dimebon displayed Ca2+-blocking properties and pronounced anticholinesterase activity. It also exhibited strong anti-NMDA ( an ion channel receptor) activity in the prevention of NMDA-induced seizures in mice.
It works by blocking L-type calcium channels that let in Calcium which destorys neurons.
Shares surged $1.75, or 29 percent, to $7.80 on the American Stock Exchange after setting a 52-week high of $8.10 earlier in the session.
Amgen's phase II results of denosumab encouraging
Amgen said results from an exploratory analysis of an ongoing phase II study showed that subjects treated with denosumab sub-Q, 60 mg twice yearly for up to 24 months , experienced enhanced bone mineral density, as well as in parameters of hip structural analysis, which estimates bone strength.
Denosumab is a fully human monoclonal antibody that targets RANK ligand, which is a primary mediator of the formation, function and survival of osteoclasts. RANK is receptor activator of Nuclear Factor kappa B, which we have talked about in an earlier post. Post NfKB activation, a diverse array of genes are expressed in osteoclasts.
AMGEN, Nasdaq:AMGN closed up $1.03, to $72.01 in heavy trading.
Denosumab is a fully human monoclonal antibody that targets RANK ligand, which is a primary mediator of the formation, function and survival of osteoclasts. RANK is receptor activator of Nuclear Factor kappa B, which we have talked about in an earlier post. Post NfKB activation, a diverse array of genes are expressed in osteoclasts.
AMGEN, Nasdaq:AMGN closed up $1.03, to $72.01 in heavy trading.
Wednesday, September 20, 2006
Looks like I would have lost that wager,
about the US markets being down today. I completely forgot about the federal reserve announcement today. The market was actually up due to the fact that interest rates won't be raised.
I have some clinical trial data from GlaxoSmith Kline and CytRX i'll post today. I have to do some more research, then i'll put up the results.
I have some clinical trial data from GlaxoSmith Kline and CytRX i'll post today. I have to do some more research, then i'll put up the results.
Tuesday, September 19, 2006
Asian markets lower after Thai military coup
HONG KONG -- Asian stocks and currencies were lower Wednesday, with the Thai baht stabilizing at 37.745 to the dollar after touching a nearly seven-month low of 37.9 overnight in the wake of a military coup in Bangkok that overthrew Prime Minister Thaksin Shinawatra.
The Thai military ordered all banks, stock exchanges and offices to remain closed Wednesday.
The overnight move in the baht was its biggest one-day decline in three years.
Analysts said the Thai economy, with more than $60 billion in foreign exchange reserves, was in a much better fiscal position to ride out the political crisis than it was nearly 10 years ago and it was unlikely there would be a bigger impact on the region.
The Asian currency crisis that erupted July 1997 began with the devaluation of the Thai baht, then snowballed into a crisis in emerging markets around the world.
Elsewhere around the region, Japan's Nikkei 225 Average (JP:1804610: news, chart, profile) was down 1.1% at 15,696.50. The broader Topix index was down 1.4% at 1,569.61. The dollar bought 117.265 yen, down from its level of 117.58 yen in late New York trading.
Australian shares also opened lower, with the S&P/ASX 200 declining 1% to 4,967.90. The Singapore Straits Times Index was down 0.8%, while South Korea's Kospi index was down 0.6%.
Shares listed in Shanghai and Malaysia also declined. New Zealand's leading share index fell 0.9%.
Taiwan's Weighted Index traded flat.
I'll put a wager on the US market is flat as well tomorrow. How long until BioTech breaks out in fall/winter trading? There's a fall chill in the air, let's go!
The Thai military ordered all banks, stock exchanges and offices to remain closed Wednesday.
The overnight move in the baht was its biggest one-day decline in three years.
Analysts said the Thai economy, with more than $60 billion in foreign exchange reserves, was in a much better fiscal position to ride out the political crisis than it was nearly 10 years ago and it was unlikely there would be a bigger impact on the region.
The Asian currency crisis that erupted July 1997 began with the devaluation of the Thai baht, then snowballed into a crisis in emerging markets around the world.
Elsewhere around the region, Japan's Nikkei 225 Average (JP:1804610: news, chart, profile) was down 1.1% at 15,696.50. The broader Topix index was down 1.4% at 1,569.61. The dollar bought 117.265 yen, down from its level of 117.58 yen in late New York trading.
Australian shares also opened lower, with the S&P/ASX 200 declining 1% to 4,967.90. The Singapore Straits Times Index was down 0.8%, while South Korea's Kospi index was down 0.6%.
Shares listed in Shanghai and Malaysia also declined. New Zealand's leading share index fell 0.9%.
Taiwan's Weighted Index traded flat.
I'll put a wager on the US market is flat as well tomorrow. How long until BioTech breaks out in fall/winter trading? There's a fall chill in the air, let's go!
Phase III GlaxoSmith-Kline Results: Boniva users Stay on Regimen Longer and have fewer Fractures
According to six-month results of ongoing studies, women with postmenopausal osteoporosis taking once-monthly Boniva® (ibandronate sodium) were approximately 25 percent more likely to stay on therapy than women taking the once-weekly medications, alendronate or risedronate.(1) To ensure the results reflect the independent effect of once-monthly dosing with Boniva, the analyses controlled for factors that could affect persistence* with therapy, including age, other medical conditions, and out-of-pocket costs for the medications -- as recommended by leading health and pharmacoeconomic research organizations.(2,3) These findings were presented today at the 28th Annual Meeting of the American Society for Bone and Mineral Research (ASBMR). The three medications studied were bisphosphonates, the most frequently prescribed class of medications for the treatment and prevention of postmenopausal osteoporosis.(4)
The findings are important in light of other presentations and previously published reports showing that staying on daily or weekly bisphosphonate therapy (persistence) may result in fewer fractures(5,6,7) and reduced healthcare costs and use of resources.(8,9,10)
"Treatment with bisphosphonates clearly reduces risk of fractures, but only if patients keep taking their medications," said lead investigator, Stuart L. Silverman, M.D., clinical professor of medicine and rheumatology at Cedars-Sinai/UCLA. He said that osteoporosis is often asymptomatic, which can reduce a patient's motivation to stay on therapy and increase their risk of debilitating fractures. "The greater persistence seen with once-monthly compared to once-weekly bisphosphonates is encouraging, particularly because the findings were consistent across two large and robust U.S. claims databases."
About the Data
These results were based on retrospective analyses of ongoing studies using two managed care databases: HealthCore and i3 Innovus, which contain prescription and health information on approximately 17.5 and 16 million enrollees, respectively.(1) These two analyses included data for 6,127 and 10,526 women who were 45 years of age or older, and who received a prescription for either once-monthly Boniva (n=277 and 1,025) or a once-weekly bisphosphonate (n=5,850 and 9,501). Patients were considered nonpersistent if they had a refill gap of 45 days or more for once-monthly Boniva, or 30 days or more for a weekly bisphosphonate. The investigators controlled for potentially confounding factors -- including age, copay and comorbidities -- as recommended by the International Society of Pharmacoeconomics and Outcomes Research and the World Health Organization.(2,3)
Compared to women who had prescriptions for a once-weekly bisphosphonate, those who had a prescription for once-monthly Boniva were 27.2 percent (p = 0.0002) and 21.7 percent (p<0.0001) more likely to persist with therapy, based on the HealthCore and i3 Innovus database analyses, respectively. The authors concluded that the increase in persistence with once-monthly Boniva was approximately 25 percent overall.(1)
This says that women on the once a month drug are more likely to stay on the medication which prevents post-menopausal osteoporosis. Women who are on daily or weekly doses of other bisphosphate drugs don't stay with the medication which results in frequent fractures and higher health care costs.
Osteoporosis is common in post-menopausal women because of the lack of hormones produced by the ovary.
In the normal bone, there are cells called osteoclasts and osteoblasts. Normally there is a lot of bone turnover, where the osteoclasts eat the bone [absorb it] and the osteoblasts creat new bone. In osteoporosis, the osteoclasts are winning the battle and absorb the bone, making it brittle.
Boniva is a bisphosphate drug, and works by inhibiting the osteoclast resorption.
GSK:NYSE closed down $1.26 today to 54.32 in heavy trading. GSK was also downgraded today to "underperform" by Bear Stearns.
The findings are important in light of other presentations and previously published reports showing that staying on daily or weekly bisphosphonate therapy (persistence) may result in fewer fractures(5,6,7) and reduced healthcare costs and use of resources.(8,9,10)
"Treatment with bisphosphonates clearly reduces risk of fractures, but only if patients keep taking their medications," said lead investigator, Stuart L. Silverman, M.D., clinical professor of medicine and rheumatology at Cedars-Sinai/UCLA. He said that osteoporosis is often asymptomatic, which can reduce a patient's motivation to stay on therapy and increase their risk of debilitating fractures. "The greater persistence seen with once-monthly compared to once-weekly bisphosphonates is encouraging, particularly because the findings were consistent across two large and robust U.S. claims databases."
About the Data
These results were based on retrospective analyses of ongoing studies using two managed care databases: HealthCore and i3 Innovus, which contain prescription and health information on approximately 17.5 and 16 million enrollees, respectively.(1) These two analyses included data for 6,127 and 10,526 women who were 45 years of age or older, and who received a prescription for either once-monthly Boniva (n=277 and 1,025) or a once-weekly bisphosphonate (n=5,850 and 9,501). Patients were considered nonpersistent if they had a refill gap of 45 days or more for once-monthly Boniva, or 30 days or more for a weekly bisphosphonate. The investigators controlled for potentially confounding factors -- including age, copay and comorbidities -- as recommended by the International Society of Pharmacoeconomics and Outcomes Research and the World Health Organization.(2,3)
Compared to women who had prescriptions for a once-weekly bisphosphonate, those who had a prescription for once-monthly Boniva were 27.2 percent (p = 0.0002) and 21.7 percent (p<0.0001) more likely to persist with therapy, based on the HealthCore and i3 Innovus database analyses, respectively. The authors concluded that the increase in persistence with once-monthly Boniva was approximately 25 percent overall.(1)
This says that women on the once a month drug are more likely to stay on the medication which prevents post-menopausal osteoporosis. Women who are on daily or weekly doses of other bisphosphate drugs don't stay with the medication which results in frequent fractures and higher health care costs.
Osteoporosis is common in post-menopausal women because of the lack of hormones produced by the ovary.
In the normal bone, there are cells called osteoclasts and osteoblasts. Normally there is a lot of bone turnover, where the osteoclasts eat the bone [absorb it] and the osteoblasts creat new bone. In osteoporosis, the osteoclasts are winning the battle and absorb the bone, making it brittle.
Boniva is a bisphosphate drug, and works by inhibiting the osteoclast resorption.
GSK:NYSE closed down $1.26 today to 54.32 in heavy trading. GSK was also downgraded today to "underperform" by Bear Stearns.
Merck Moves forward with Vioxx Successor--Arcoxia
Merck Looks Forward To Reviewing The MEDAL Program Data With Regulatory Agencies And Intends To Respond To FDA-Issued ''Approvable'' Letter
Merck & Co., Inc. announced today that preliminary analyses indicate the MEDAL (Multinational Etoricoxib and Diclofenac Arthritis Long-Term) Program showed that the rate of confirmed thrombotic cardiovascular (CV) events was similar between the selective COX-2 inhibitor ARCOXIA(TM) and diclofenac, a traditional nonsteroidal anti-inflammatory drug (NSAID). Specifically, in the pre-specified "per-protocol" analysis of the primary endpoint, the relative risk of confirmed thrombotic CV events between ARCOXIA and diclofenac was 0.95 (95 percent CI: 0.81, 1.11). In the "intent-to-treat" analysis, the relative risk of confirmed thrombotic CV events between ARCOXIA and diclofenac was 1.05 (95 percent CI: 0.93, 1.19), consistent with the primary per-protocol analysis.
Merck is seeking indications for Arcoxia for the treatment of osteoarthritis, rheumatoid arthritis, chronic low back pain, acute pain, dysmenorrhea (menstrual pain), acute gouty arthritis and ankylosing spondylitis. Arcoxia is a selective COX-2 inhibitor (cyclooxygenase 2- an enzyme that is involved in the inflammatory pathway.) This drug is a NSAID, non steroidal anti inflammatory drug, like Advil. It selectively inhibits COX-2 and not COX-1 so that the intestinal tract isnt irritated.
I'll post the heart related data a little later, pretty interesting. Looks really safe.
Merck closed today up 0.38 cents to $41.18
Merck & Co., Inc. announced today that preliminary analyses indicate the MEDAL (Multinational Etoricoxib and Diclofenac Arthritis Long-Term) Program showed that the rate of confirmed thrombotic cardiovascular (CV) events was similar between the selective COX-2 inhibitor ARCOXIA(TM) and diclofenac, a traditional nonsteroidal anti-inflammatory drug (NSAID). Specifically, in the pre-specified "per-protocol" analysis of the primary endpoint, the relative risk of confirmed thrombotic CV events between ARCOXIA and diclofenac was 0.95 (95 percent CI: 0.81, 1.11). In the "intent-to-treat" analysis, the relative risk of confirmed thrombotic CV events between ARCOXIA and diclofenac was 1.05 (95 percent CI: 0.93, 1.19), consistent with the primary per-protocol analysis.
Merck is seeking indications for Arcoxia for the treatment of osteoarthritis, rheumatoid arthritis, chronic low back pain, acute pain, dysmenorrhea (menstrual pain), acute gouty arthritis and ankylosing spondylitis. Arcoxia is a selective COX-2 inhibitor (cyclooxygenase 2- an enzyme that is involved in the inflammatory pathway.) This drug is a NSAID, non steroidal anti inflammatory drug, like Advil. It selectively inhibits COX-2 and not COX-1 so that the intestinal tract isnt irritated.
I'll post the heart related data a little later, pretty interesting. Looks really safe.
Merck closed today up 0.38 cents to $41.18
Breaking Cancer News: Patent in Imclone Case Is Israelis
NEW YORK - In a blow to ImClone Systems Inc., a federal judge ruled Monday that three scientists from Israel are the true inventors of a patent used for the company's blockbuster cancer drug, Erbitux, rather than seven people whose names are now on it.
U.S. District Judge Naomi Reice Buchwald made the finding in a 140-page opinion that said lawyers for the scientists had proved they were entitled to sole inventorship of the patent.
Lawyers had predicted that the case could have significant effects on the future of Erbitux, a colon-cancer treatment drug made by ImClone, the company whose founder, Sam Waksal, is serving a prison sentence for his role in the stock scandal that also ensnared Martha Stewart. [Remember Ms. Stewart did not go to prison for this, she went for lying to a grand jury]
In a 2003 lawsuit, Yeda Research and Development Co. of Israel sued ImClone, which has an exclusive license for the formula used in Erbitux to inhibit tumor cells, and its partner Aventis, claiming three of its researchers were the real inventors.
During hearings this summer, plaintiffs' lawyer Nicholas Groombridge said ImClone could lose its exclusivity with the technique covered by the license if Yeda's scientists were credited. He said a ruling such as the judge's on Monday would free Yeda to license the patent to other drug companies. He said if all the names currently on the patent were taken off, ImClone would not have a license anymore.
"I presume there would be a negotiation and a deal would be reached," he said then. "It is not the intent of Yeda to keep anybody off the market."
A lawyer for Aventis later noted that one trial witness had remarked that hundreds of millions of dollars were at stake for Yeda and Erbitux, which is distributed in the U.S. by Bristol-Myers Squibb Co.
Imclone trades on the NASDAQ and is down $1.93 to $28.60 in early afternoon trading.
U.S. District Judge Naomi Reice Buchwald made the finding in a 140-page opinion that said lawyers for the scientists had proved they were entitled to sole inventorship of the patent.
Lawyers had predicted that the case could have significant effects on the future of Erbitux, a colon-cancer treatment drug made by ImClone, the company whose founder, Sam Waksal, is serving a prison sentence for his role in the stock scandal that also ensnared Martha Stewart. [Remember Ms. Stewart did not go to prison for this, she went for lying to a grand jury]
In a 2003 lawsuit, Yeda Research and Development Co. of Israel sued ImClone, which has an exclusive license for the formula used in Erbitux to inhibit tumor cells, and its partner Aventis, claiming three of its researchers were the real inventors.
During hearings this summer, plaintiffs' lawyer Nicholas Groombridge said ImClone could lose its exclusivity with the technique covered by the license if Yeda's scientists were credited. He said a ruling such as the judge's on Monday would free Yeda to license the patent to other drug companies. He said if all the names currently on the patent were taken off, ImClone would not have a license anymore.
"I presume there would be a negotiation and a deal would be reached," he said then. "It is not the intent of Yeda to keep anybody off the market."
A lawyer for Aventis later noted that one trial witness had remarked that hundreds of millions of dollars were at stake for Yeda and Erbitux, which is distributed in the U.S. by Bristol-Myers Squibb Co.
Imclone trades on the NASDAQ and is down $1.93 to $28.60 in early afternoon trading.
Tuesday's Word: Coup
Apparently, as you all know, there is an attempted coup in Thailand. And if you have seen the US market, investors are reacting by keeping their hands in their pockets. The NYSE is down 55.55 in early afternoon trading.
I'll update periodically today if any biotech-pharma companies release any data.
I'll update periodically today if any biotech-pharma companies release any data.
Monday, September 18, 2006
Monday: Word of the Day.....Methylation
Methylation occurs naturally all the time in the mammalian genome. In terms of humans, it is the addition of a CH3 group to DNA. This then inhibits the DNA from replication. Methylation is a fundamental way of how the cell regulates DNA replication. There are specific enzymes [DNA methylases] that regulate this process.
Orion Genomics is a company working on DNA methylation modifying drugs.
Orion Genomics is a company working on DNA methylation modifying drugs.
Gemin X Biotechnologies Initiates Phase 2 Clinical Trial Program for GX15-070 in Myelofibrosis and Hodgkin's Lymphoma
Gemin X Biotechnologies Inc. announced today the initiation of a Phase 2 clinical trial program to evaluate GX15-070 in the treatment of several types of cancer. GX15-070 is a small molecule specifically designed to inhibit all relevant members of the Bcl-2 protein family, a validated cancer target, thus restoring the natural cell death process of apoptosis.
Phase 2 trials have been initiated in Hodgkin's Lymphoma and in myelofibrosis with myeloid metaplasia. Both trials are multi-center, open-label, single agent studies that will be conducted in North America at the M.D. Anderson Cancer Center and other locations. The Hodgkin's Lymphoma trial will enroll 10-29 patients and the myelofibrosis trial will enroll 19-55 patients. The primary endpoint of the trials is tumor response, and safety, pharmacokinetics and pharmacodynamics will also be measured.
In Phase 1 studies reported to date, GX15-070 was well-tolerated and resulted in clinical and biological activity.
Results from a third study in hematological malignancies are expected to be presented at the American Society of Hematology (ASH) annual meeting this coming December.
What does all that mean? Let's take this step by step and figure it out. Bcl-2 family members are proteins inside cells that are involved in apoptosis (programmed cell death). For example, cells sense their environment or receive stimuli, and can decide to kill themselves. It is a very regulated process that proceeds step by step until rupture of the membrane. GX15 is targeted to the protein Bcl-2, which when it gets activated, pushes the cell towards apoptosis. The cool thing is that the drug inhibits Bcl-2 (and all it's family members), AND activates proteins such as Bax and Bak, which give a survival signal to the cell.
Lymphomas are immune cell cancers. Myelofibrosis is a disorder of the bone marrow where the marrow is replaced by fibrosis (scar tissue).
Gemin X is a private company in Montreal, Canada.
Phase 2 trials have been initiated in Hodgkin's Lymphoma and in myelofibrosis with myeloid metaplasia. Both trials are multi-center, open-label, single agent studies that will be conducted in North America at the M.D. Anderson Cancer Center and other locations. The Hodgkin's Lymphoma trial will enroll 10-29 patients and the myelofibrosis trial will enroll 19-55 patients. The primary endpoint of the trials is tumor response, and safety, pharmacokinetics and pharmacodynamics will also be measured.
In Phase 1 studies reported to date, GX15-070 was well-tolerated and resulted in clinical and biological activity.
Results from a third study in hematological malignancies are expected to be presented at the American Society of Hematology (ASH) annual meeting this coming December.
What does all that mean? Let's take this step by step and figure it out. Bcl-2 family members are proteins inside cells that are involved in apoptosis (programmed cell death). For example, cells sense their environment or receive stimuli, and can decide to kill themselves. It is a very regulated process that proceeds step by step until rupture of the membrane. GX15 is targeted to the protein Bcl-2, which when it gets activated, pushes the cell towards apoptosis. The cool thing is that the drug inhibits Bcl-2 (and all it's family members), AND activates proteins such as Bax and Bak, which give a survival signal to the cell.
Lymphomas are immune cell cancers. Myelofibrosis is a disorder of the bone marrow where the marrow is replaced by fibrosis (scar tissue).
Gemin X is a private company in Montreal, Canada.
Generex Biotechnology Announces U.S. Distribution of New Glucose Rapidspray(TM) Product
Generex Biotechnology Announces U.S. Distribution of New Glucose Rapidspray(TM) Product
TORONTO -- Generex Biotechnology Corporation the leader in metabolic diseases drug delivery through the inner lining of the mouth, announced today that Cardinal Health will distribute its new Glucose RapidSpray™ product in the U.S.
Glucose RapidSpray is expected to be in stores by October, 2006.
Glucose RapidSpray is an innovative alternative for people who require or want additional glucose in their diet. Glucose RapidSpray delivers a fat-free, low-calorie glucose formulation that was developed using the Company's proprietary buccal drug delivery technologies. Glucose RapidSpray delivers glucose directly into the mouth where the proprietary formulation is rapidly absorbed into the blood stream. Glucose RapidSpray is simple to carry and use, with no large tablets to chew or messy gels to swallow.
"Using our patented technology -- RapidMist™ -- we've developed a product that will deliver glucose to anyone who needs a fast and efficient means for receiving glucose," said Anna Gluskin, President & Chief Executive Officer of Generex. "We expect that Glucose RapidSpray will appeal to people battling the symptoms of low blood sugar who now use other over-the-counter glucose products but want a faster-acting product. We expect to position Glucose RapidSpray as a companion product to Generex Oral-lyn™, our proprietary oral insulin spray product, in the diabetes management field."
Transmucosal glucose treatment is already used for diabetes without the discomfort of needles and pulmonary inhalation (lung). I'd like to know what the RapidSpray formulation is....
Generex trades on the NASDAQ: GNBT, and is up today 17% to $1.67.
TORONTO -- Generex Biotechnology Corporation the leader in metabolic diseases drug delivery through the inner lining of the mouth, announced today that Cardinal Health will distribute its new Glucose RapidSpray™ product in the U.S.
Glucose RapidSpray is expected to be in stores by October, 2006.
Glucose RapidSpray is an innovative alternative for people who require or want additional glucose in their diet. Glucose RapidSpray delivers a fat-free, low-calorie glucose formulation that was developed using the Company's proprietary buccal drug delivery technologies. Glucose RapidSpray delivers glucose directly into the mouth where the proprietary formulation is rapidly absorbed into the blood stream. Glucose RapidSpray is simple to carry and use, with no large tablets to chew or messy gels to swallow.
"Using our patented technology -- RapidMist™ -- we've developed a product that will deliver glucose to anyone who needs a fast and efficient means for receiving glucose," said Anna Gluskin, President & Chief Executive Officer of Generex. "We expect that Glucose RapidSpray will appeal to people battling the symptoms of low blood sugar who now use other over-the-counter glucose products but want a faster-acting product. We expect to position Glucose RapidSpray as a companion product to Generex Oral-lyn™, our proprietary oral insulin spray product, in the diabetes management field."
Transmucosal glucose treatment is already used for diabetes without the discomfort of needles and pulmonary inhalation (lung). I'd like to know what the RapidSpray formulation is....
Generex trades on the NASDAQ: GNBT, and is up today 17% to $1.67.
Phase III Study Initiated for Genmab's EGF Antibody
Genmab A/S began a pivotal study of HuMax-EGFR (zalutumumab) in otherwise incurable patients with head and neck cancer, an indication that could serve as a springboard for using the human antibody more down the road.
Starting in patients who are very sick and have no other alternative the company plans to expand the product's use into first line head and neck cancer treatment and other diseases.
Patients in the study will receive an initial dose of 8mg/kg, followed by weekly infusions of maintenace doses and disease progression followed until death.
EGF stands for epidermal growth factor and tumor cells need this to sustain growth. Drugs like this are out on the market now, eg, Erbitux from Imclone, but are indicated for colon cancers.
The Copenhagen, Denmark based company saw its shares on the Copenhagen Stock Exchange (CSE:GEN lose DKK2 to close at DKK224 or $38.21. It is traded in the US as GNMSF.PK and is up to $38.25
Starting in patients who are very sick and have no other alternative the company plans to expand the product's use into first line head and neck cancer treatment and other diseases.
Patients in the study will receive an initial dose of 8mg/kg, followed by weekly infusions of maintenace doses and disease progression followed until death.
EGF stands for epidermal growth factor and tumor cells need this to sustain growth. Drugs like this are out on the market now, eg, Erbitux from Imclone, but are indicated for colon cancers.
The Copenhagen, Denmark based company saw its shares on the Copenhagen Stock Exchange (CSE:GEN lose DKK2 to close at DKK224 or $38.21. It is traded in the US as GNMSF.PK and is up to $38.25
Sunday, September 17, 2006
Schering-Plough CEO Cures Host of Ills
KENILWORTH, N.J. (AP) -- Fred Hassan calls running Schering-Plough Corp. the toughest job he's ever had: taking a drug company in critical condition, treating its ills simultaneously and restoring health.
ADVERTISEMENT
But within 3 1/2 years, the chief executive has managed to put Schering-Plough back in the black, nudge up its stock price and cure most of the disorders he inherited from prior management. Those included slumping sales with little prospect of lucrative new drugs, a demoralized work force and multiple government probes of corporate wrongdoing and manufacturing violations.
Hassan is so well regarded as a turnaround specialist that speculation surfaced Friday Schering-Plough might try to merge with New York-based Bristol-Myers Squibb Co., which booted its CEO Tuesday. Neither company would comment on that possibility.
Schering-Plough makes prescription respiratory, cholesterol and hepatitis drugs and consumer items such as Coppertone sun care lotions, Claritin allergy pills and Dr. Scholl's foot care products. The company has been on the rebound since Hassan, a Harvard-trained MBA from Pakistan, took over.
thanks to yahoo for the article: http://biz.yahoo.com/ap/060917/schering_plough_revival.html?.v=4
ADVERTISEMENT
But within 3 1/2 years, the chief executive has managed to put Schering-Plough back in the black, nudge up its stock price and cure most of the disorders he inherited from prior management. Those included slumping sales with little prospect of lucrative new drugs, a demoralized work force and multiple government probes of corporate wrongdoing and manufacturing violations.
Hassan is so well regarded as a turnaround specialist that speculation surfaced Friday Schering-Plough might try to merge with New York-based Bristol-Myers Squibb Co., which booted its CEO Tuesday. Neither company would comment on that possibility.
Schering-Plough makes prescription respiratory, cholesterol and hepatitis drugs and consumer items such as Coppertone sun care lotions, Claritin allergy pills and Dr. Scholl's foot care products. The company has been on the rebound since Hassan, a Harvard-trained MBA from Pakistan, took over.
thanks to yahoo for the article: http://biz.yahoo.com/ap/060917/schering_plough_revival.html?.v=4
Saturday, September 16, 2006
Word of the Day, Saturday: Pubmed
Pubmed is a scientific literature search engine. You can search for particular disease, authors and key words. It's an invaluable tool for reviewing the literature. I always have a window open with pubmed running. So next time you need a publication, check it out.
Common Sense: Most Money Is Made By Waiting, Not Trading
Most Money Is Made By Waiting, Not Trading
A common mistake for stock market investors is overtrading. Learning to sit and wait is one of the biggest challenges for many.
Jesse Livermore, one of the world's greatest investors, said: "It is never your thinking that makes big money. It's the sitting."
Stocks stair-step their way higher by breaking out of basing patterns, running up and then forming new bases from which they break out. Savvy investors learn how to hang on to leading stocks that go through normal pullbacks rather than sell them.
Beginning investors are often shaken out of winning stocks because they buy incorrectly. You can avoid that by buying a stock within 5% of its buy point in a sound base. Studies of past winners show that stocks breaking out of sound bases on heavy volume rarely retreat more than 8% from the proper buy point.
So buying right will let you sit longer with winners.
They don't all work, of course. So you should always sell if a stock falls 8% below your buy point. That saves your capital for stocks that do work.
After a substantial run-up, a correction of 8% to 12% from a peak is considered normal. If you buy a stock on a breakout and it goes up 20%, don't sell just because it pulls back from its high. Watch to see if it can resume its run or carve out a new base.
Just be careful not to let such gains turn into losses by holding on as a stock falls back below the original buy point.
And keep an eye out for stocks with extra oomph. One that shoots up 20% or more in only one to four weeks should be held for at least eight weeks unless it falls all the way back near your buy point.
Stocks that show this kind of strength often double or triple in short order. But they often correct sharply, shaking out those who don't follow the eight-week rule.
A common mistake for stock market investors is overtrading. Learning to sit and wait is one of the biggest challenges for many.
Jesse Livermore, one of the world's greatest investors, said: "It is never your thinking that makes big money. It's the sitting."
Stocks stair-step their way higher by breaking out of basing patterns, running up and then forming new bases from which they break out. Savvy investors learn how to hang on to leading stocks that go through normal pullbacks rather than sell them.
Beginning investors are often shaken out of winning stocks because they buy incorrectly. You can avoid that by buying a stock within 5% of its buy point in a sound base. Studies of past winners show that stocks breaking out of sound bases on heavy volume rarely retreat more than 8% from the proper buy point.
So buying right will let you sit longer with winners.
They don't all work, of course. So you should always sell if a stock falls 8% below your buy point. That saves your capital for stocks that do work.
After a substantial run-up, a correction of 8% to 12% from a peak is considered normal. If you buy a stock on a breakout and it goes up 20%, don't sell just because it pulls back from its high. Watch to see if it can resume its run or carve out a new base.
Just be careful not to let such gains turn into losses by holding on as a stock falls back below the original buy point.
And keep an eye out for stocks with extra oomph. One that shoots up 20% or more in only one to four weeks should be held for at least eight weeks unless it falls all the way back near your buy point.
Stocks that show this kind of strength often double or triple in short order. But they often correct sharply, shaking out those who don't follow the eight-week rule.
Friday, September 15, 2006
Lesson of the day: Friday
Don't let the market hold your money over the weekend. I got burned........
Critical Therapeutics Drug Data Weak: Read here to find out why
Shares of biotech drugmaker Critical Therapeutics Inc. set a fresh 52-week low Friday after the company said a mid-stage clinical trial for an anti-inflammatory compound that was stopped early showed no trends that the drug was effective.
The company said the Phase II trial looked to see if the compound CTI-01 was effective in reducing complications 14 to 28 days after heart surgery. However, the study was halted in March after a manufacturing issue arose surrounding the drug's container closure.
Only 102 patients had been enrolled, not enough to show statistical significance for efficacy. But even data from these patients did not show a trend toward effectiveness, the company said.
Critical Therapeutics plans to assess if there is an opportunity to continue development of CTI-01 with a collaborative partner, or to out-license it.
Shares of Critical Therapeutics fell 12 cents, or 4.3 percent, to $2.70 in morning trading on the Nasdaq. Earlier in the session, the stock set a new 52-week low of $2.56. The previous low of $2.73 was set Thursday.
CTI-01 is Ethyl pyruvate. Pyruvate plays a role in metabolism as a free radical scavenger. However, it cannot be useful in the cell alone because it is not stable. Therefore the company has modified it chemically for stability. it is a strong antiinflammatory agent being used in numerous treatments such as ischemic heart disease (heart attack in the ER, stroke and diabetes. There was no difference in CTI-01 from placebo. More than likely it's a stability problem or dosing issue.
Questions? Feel free to ask and I'll answer them the best I can.
The company said the Phase II trial looked to see if the compound CTI-01 was effective in reducing complications 14 to 28 days after heart surgery. However, the study was halted in March after a manufacturing issue arose surrounding the drug's container closure.
Only 102 patients had been enrolled, not enough to show statistical significance for efficacy. But even data from these patients did not show a trend toward effectiveness, the company said.
Critical Therapeutics plans to assess if there is an opportunity to continue development of CTI-01 with a collaborative partner, or to out-license it.
Shares of Critical Therapeutics fell 12 cents, or 4.3 percent, to $2.70 in morning trading on the Nasdaq. Earlier in the session, the stock set a new 52-week low of $2.56. The previous low of $2.73 was set Thursday.
CTI-01 is Ethyl pyruvate. Pyruvate plays a role in metabolism as a free radical scavenger. However, it cannot be useful in the cell alone because it is not stable. Therefore the company has modified it chemically for stability. it is a strong antiinflammatory agent being used in numerous treatments such as ischemic heart disease (heart attack in the ER, stroke and diabetes. There was no difference in CTI-01 from placebo. More than likely it's a stability problem or dosing issue.
Questions? Feel free to ask and I'll answer them the best I can.
Thursday, September 14, 2006
BioTech vs. Academia: Why can't we get along?
I have worked in both biotech and academia and heard this argument for years. There is a clear jealous element there from academia (that's the least offensive word I could come up with) on the money that industrial scientists earn. Whilst those in academia say that industrial science get too focused on one thing and "not the big picture". The argument continues in that for all your successful work in academic science, you have to beg for money every four years [depending on the mood of the NIH], and IF your fruits payoff, your reward is just a published paper. On the other hand, if you have an active role in biotech research, you may have contributed to a life saving drug. To me, that beats a paper in some political biased journal---[remember it's not what you know but who.] I think that the bias against Biotech is that academia is pretty much set in their position--you do a postdoc, get your own lab, and publish or perish. If successful, then you get tenure and continue your basic research on flies/yeast/slime mold. What is the HUMAN PHYSIOLOGICAL SIGNIFICANCE OF YOUR RESEARCH is what you have to ask yourself.
Industry relies on your basic research for initial development/background analysis of future IND development. The scrutiny that Biotech science goes through would clearly not function in the University setting.
In the end, it's not practical to argue; it's apples and oranges when you consider both sides.
I'd like to read your opinions.
Industry relies on your basic research for initial development/background analysis of future IND development. The scrutiny that Biotech science goes through would clearly not function in the University setting.
In the end, it's not practical to argue; it's apples and oranges when you consider both sides.
I'd like to read your opinions.
Apotex deal shows impact of generics on Pharma
Bristol-Myers Squibb and Sanofi-Aventis troubled deal with Apotex highlights the tactics that traditional large pharmaceutical companies are being forced to adopt as they struggle to adjust to intensifying international competition from generic medicines.
From friendly deals with, or even takeovers of, their generic rivals, through to bitter litigation or price cutting to fight against them, they are under heavy pressure to adapt to a more hostile commercial environment. While pharmaceuticals remain highly profitable, the escalating value of drug sales – more than $600bn globally last year – is creating an ever heavier burden on health systems.
Although drugs represent a small proportion of total health expenditures, they are an easier target than many others – such as doctors' salaries, hospital overheads and older more expensive forms of care.
n the period 2005-2010, Datamonitor, the market research company, estimates that the pharmaceutical industry is threatened by the loss of $105bn in revenues from medicines that are coming off patent.
However, the pace of scientific research and drug development is failing to keep pace, with the number of new patented medicines well below past levels.
Companies are experimenting with new management techniques to boost efficiency in innovation as they seek to generate the next generation of drugs.
In the US, legislation encourages generic companies to challenge drug company patents, with the incentive of exclusivity for the first six months after launch. Health reforms introduced last year that indirectly make the federal government the purchaser of the majority of drugs are bringing fresh pressures for efficiency.
In Europe, no such rules exist, but some countries such as Germany strongly favour the generic industry, and all of the state-funded health systems are seeking ways to cut their drug bills.
Novartis, the research-based Swiss-based group, went as far as to buy the German business Hexal last year, becoming one of the world's largest generic companies as a result.
But competition is not limited to the patented drug companies. "The volume of our business is increasing, but the pricing is not," says Greg Perry, head of the European Generics Association, a trade body.
With low-cost generic companies in the developing world – such as Ranbaxy in India – expanding into Europe and the US, there is a wave of consolidation within the generic industry too.
This is why drug companies need revenue to discover and market next generation medicines.
Bristol-Myers Squibb trades on the New York Stock Exchange under the symbol BMY and was down 0.13 cents/share. Aventis-Sanofi trades on the NYSE under SNY and lost 0.47 cents to 43.38.
From friendly deals with, or even takeovers of, their generic rivals, through to bitter litigation or price cutting to fight against them, they are under heavy pressure to adapt to a more hostile commercial environment. While pharmaceuticals remain highly profitable, the escalating value of drug sales – more than $600bn globally last year – is creating an ever heavier burden on health systems.
Although drugs represent a small proportion of total health expenditures, they are an easier target than many others – such as doctors' salaries, hospital overheads and older more expensive forms of care.
n the period 2005-2010, Datamonitor, the market research company, estimates that the pharmaceutical industry is threatened by the loss of $105bn in revenues from medicines that are coming off patent.
However, the pace of scientific research and drug development is failing to keep pace, with the number of new patented medicines well below past levels.
Companies are experimenting with new management techniques to boost efficiency in innovation as they seek to generate the next generation of drugs.
In the US, legislation encourages generic companies to challenge drug company patents, with the incentive of exclusivity for the first six months after launch. Health reforms introduced last year that indirectly make the federal government the purchaser of the majority of drugs are bringing fresh pressures for efficiency.
In Europe, no such rules exist, but some countries such as Germany strongly favour the generic industry, and all of the state-funded health systems are seeking ways to cut their drug bills.
Novartis, the research-based Swiss-based group, went as far as to buy the German business Hexal last year, becoming one of the world's largest generic companies as a result.
But competition is not limited to the patented drug companies. "The volume of our business is increasing, but the pricing is not," says Greg Perry, head of the European Generics Association, a trade body.
With low-cost generic companies in the developing world – such as Ranbaxy in India – expanding into Europe and the US, there is a wave of consolidation within the generic industry too.
This is why drug companies need revenue to discover and market next generation medicines.
Bristol-Myers Squibb trades on the New York Stock Exchange under the symbol BMY and was down 0.13 cents/share. Aventis-Sanofi trades on the NYSE under SNY and lost 0.47 cents to 43.38.
Thursday word of the day: Efficacy
I see this word thrown around a lot in biotech company press releases, usually incorrectly. Usually it gets mixed up with synergistic. So let's settle it.
EFFICACY: (Of a drug or treatment). The maximum ability of a drug or treatment to produce a result regardless of dosage. A drug passes efficacy trials if it is effective at the dose tested and against the illness for which it is prescribed. In the procedure mandated by the FDA, Phase II clinical trials gauge efficacy, and Phase III trials confirm it
EFFICACY: (Of a drug or treatment). The maximum ability of a drug or treatment to produce a result regardless of dosage. A drug passes efficacy trials if it is effective at the dose tested and against the illness for which it is prescribed. In the procedure mandated by the FDA, Phase II clinical trials gauge efficacy, and Phase III trials confirm it
An explanation of Campath from Genzyme
Genzyme today announced a two-year interim results from a Phase 2 trial comparing Campath® (alemtuzumab) with Rebif® (interferon beta-1a) for the treatment of multiple sclerosis. The results derive from a pre-specified analysis conducted after two years of treatment for 334 patients in the planned three-year trial. This review was conducted in conjunction with an independent data and safety monitoring board.
As previously announced, dosing of alemtuzumab in this study was suspended in September 2005 after three patients developed immune thrombocytopenic purpura (ITP), a treatable condition in which patients experience a low platelet count as a result of an immune response directed against the platelets. At that time, most patients had received two cycles of therapy with alemtuzumab. Treatment with Rebif in the control arm has continued without interruption. The trial remains on clinical hold in the United States, and Genzyme is working closely with clinical investigators and regulatory agencies to complete the study and ensure that the risk of ITP is well understood and managed. The company discourages physicians and patients from using alemtuzumab for MS outside of a clinical trial setting in which procedures are in place for managing ITP risk.
The rest of the story is here: http://biz.yahoo.com/prnews/060914/neth013.html?.v=71
What happenend from the scientific point of view? What is interferon beta1? What is Campath? What are platelets? Time to find out!
First of all Campath is used in the treatment of multiple sclerosis. It is a monoclonal humanized antibody (it's name is alemtuzumab---you can always quickly what drugs are antibodies quick; the mab at the end of the word means "monoclonal antibody")that recognizes CD52 on immune system tumor cells. OK, what is CD52? CD52 is a tumor antigen. When Campath binds to CD52, the body recognizes it as disease and proceeds to kill cells with Campath bound. It does this by antibody dependent cytotoxicity and complement.
Interferon is a proinflammaotry cytokine released by cells to trigger the immune response. This helps the body determine which cells to kill that are diseased. It is a strong immuno-therepy drug used for many diseases including viral infections.
If you have any more specific questions I didn't get into here, leave me a comment and i'll try to explain it in another post.
Genzyme GeNZ closed NASDAQ trading today up 2.7% at 68.35
As previously announced, dosing of alemtuzumab in this study was suspended in September 2005 after three patients developed immune thrombocytopenic purpura (ITP), a treatable condition in which patients experience a low platelet count as a result of an immune response directed against the platelets. At that time, most patients had received two cycles of therapy with alemtuzumab. Treatment with Rebif in the control arm has continued without interruption. The trial remains on clinical hold in the United States, and Genzyme is working closely with clinical investigators and regulatory agencies to complete the study and ensure that the risk of ITP is well understood and managed. The company discourages physicians and patients from using alemtuzumab for MS outside of a clinical trial setting in which procedures are in place for managing ITP risk.
The rest of the story is here: http://biz.yahoo.com/prnews/060914/neth013.html?.v=71
What happenend from the scientific point of view? What is interferon beta1? What is Campath? What are platelets? Time to find out!
First of all Campath is used in the treatment of multiple sclerosis. It is a monoclonal humanized antibody (it's name is alemtuzumab---you can always quickly what drugs are antibodies quick; the mab at the end of the word means "monoclonal antibody")that recognizes CD52 on immune system tumor cells. OK, what is CD52? CD52 is a tumor antigen. When Campath binds to CD52, the body recognizes it as disease and proceeds to kill cells with Campath bound. It does this by antibody dependent cytotoxicity and complement.
Interferon is a proinflammaotry cytokine released by cells to trigger the immune response. This helps the body determine which cells to kill that are diseased. It is a strong immuno-therepy drug used for many diseases including viral infections.
If you have any more specific questions I didn't get into here, leave me a comment and i'll try to explain it in another post.
Genzyme GeNZ closed NASDAQ trading today up 2.7% at 68.35
Wednesday, September 13, 2006
Merck Challenged about validity of Vioxx Clinical Trials
Two studies offer more evidence about the dangers of some painkillers, adding kidney problems to heart concerns already raised with the drug once sold as Vioxx, researchers said.
One report from Boston's Brigham and Women's Hospital and Harvard Medical School said an analysis of 114 studies involving more than 116,000 people showed that rofecoxib (the chemical name for Vioxx) "was associated with increased renal and (heart) arrhythmia risks."
Why the drug would cause kidney damage is unclear, it added.
Merck & Co Inc. withdrew Vioxx from the market in September 2004 after a three-year study showed it doubled the risk of heart attack and strokes in patients taking it for at least 18 months.
A second report from the University of Newcastle, New South Wales, Australia, said a look at 23 studies confirms findings of an increased risk of heart problems with Vioxx that could be found "during the first 30 days of treatment. This conclusion is consistent with a recent reanalysis ... which contradicts the original suggestion that the vascular risk was only seen after 18 months."
Merck is facing more than 11,500 product liability lawsuits from people claiming to have been harmed by Vioxx.
"Those studies will also be used to cross-examine and impeach Merck's experts who testify that there is no link between the drug and the injuries," McClellan said. "The impact could be profound in the outcomes of the trials."
Merck said it still believes the data confirm the increased heart risk begins only after the medicine had been taken for 18 months.
The Australian analysis also found that celecoxib -- sold as Celebrex by Pfizer Inc. -- was not associated with heart problems at a dose no greater than 200 milligrams a day.
In his editorial, Graham said the studies demonstrate that Vioxx "increases the risk of acute myocardial infarction at low and high doses" and that "there is no initial 18-month period of immunity from risk."
He said Celebrex increases heart risk at doses higher than 200 milligrams per day and several other non-steroidal anti-inflammatory drugs (NSAIDs) increase risk, including diclofenac, meloxicam, indomethacin and "probably" ibuprofen, while studies agree naproxen is "neutral" for heart attack risk.
Graham added that for most patients with arthritis or other conditions requiring chronic pain relief "naproxen appears to be the safest NSAID choice from a cardiovascular perspective." Naproxen is commonly sold as Aleve by Bayer Corp..
I read the JAMA science article, the data is quite clear to me.
Merck closed today at $41.09, down 2.5% on NYSE trading.
One report from Boston's Brigham and Women's Hospital and Harvard Medical School said an analysis of 114 studies involving more than 116,000 people showed that rofecoxib (the chemical name for Vioxx) "was associated with increased renal and (heart) arrhythmia risks."
Why the drug would cause kidney damage is unclear, it added.
Merck & Co Inc. withdrew Vioxx from the market in September 2004 after a three-year study showed it doubled the risk of heart attack and strokes in patients taking it for at least 18 months.
A second report from the University of Newcastle, New South Wales, Australia, said a look at 23 studies confirms findings of an increased risk of heart problems with Vioxx that could be found "during the first 30 days of treatment. This conclusion is consistent with a recent reanalysis ... which contradicts the original suggestion that the vascular risk was only seen after 18 months."
Merck is facing more than 11,500 product liability lawsuits from people claiming to have been harmed by Vioxx.
"Those studies will also be used to cross-examine and impeach Merck's experts who testify that there is no link between the drug and the injuries," McClellan said. "The impact could be profound in the outcomes of the trials."
Merck said it still believes the data confirm the increased heart risk begins only after the medicine had been taken for 18 months.
The Australian analysis also found that celecoxib -- sold as Celebrex by Pfizer Inc. -- was not associated with heart problems at a dose no greater than 200 milligrams a day.
In his editorial, Graham said the studies demonstrate that Vioxx "increases the risk of acute myocardial infarction at low and high doses" and that "there is no initial 18-month period of immunity from risk."
He said Celebrex increases heart risk at doses higher than 200 milligrams per day and several other non-steroidal anti-inflammatory drugs (NSAIDs) increase risk, including diclofenac, meloxicam, indomethacin and "probably" ibuprofen, while studies agree naproxen is "neutral" for heart attack risk.
Graham added that for most patients with arthritis or other conditions requiring chronic pain relief "naproxen appears to be the safest NSAID choice from a cardiovascular perspective." Naproxen is commonly sold as Aleve by Bayer Corp..
I read the JAMA science article, the data is quite clear to me.
Merck closed today at $41.09, down 2.5% on NYSE trading.
Wednesday Word of the day: Pharmacokinetics
PHARMACOKINETICS: The processes (in a living organism) of absorption, distribution, metabolism, and excretion of a drug or vaccine.
This is one of the first things done in a drug investigation. It goes hand in hand with toxicity. Companies pay A LOT of money to do these studies, so they better be right when the drug hits the clinic.
This is one of the first things done in a drug investigation. It goes hand in hand with toxicity. Companies pay A LOT of money to do these studies, so they better be right when the drug hits the clinic.
Cardiome shares drop on heart drug test data
TORONTO--Cardiome Pharma Corp.'s shares dipped more than 10 percent on Wednesday after reporting mixed phase 2a results for a treatment for abnormal heartbeats, which fell short of investor expectations.
The study of the investigational drug RSD1235 for the conversion of atrial fibrillation showed that 61 percent of patients in the 300 mg dose completed the study with a normal heart rhythm, compared with 43 percent of all patients receiving a placebo.
Patients in the 600 mg dosing group also showed a 61 percent occurrence of normal heart rhythm compared with 43 percent of patients receiving a placebo, but fell short of expectations on a key comparison.
The data was in line with similar preliminary data Cardiome reported in late July, which sent the stock soaring almost 35 percent in one day after the numbers were released.
Cardiome shares were down C$1.67, or 11.2 percent, at C$13.27 on the Toronto Stock Exchange at midday on a volume of 429,000 shares. On Nasdaq, the shares were off $1.50, or 11.3 percent, at $11.81.
Analysts said investors were looking for improved results above the reported 61 percent in the 600 mg range, from the latest tests.
"This is a continuation of the interim results. I just think that the market got overly enthusiastic with the interim results and we were looking for something much better," said John Maletic, an analyst at Scotia Capital in Toronto.
The Vancouver, British Columbia-based company said 171 patients were successfully cardioverted after the initial three days of dosing and continued in the study, of which 159 either completed the dosing or relapsed to atrial fibrillation.
The remainder of the patients were discontinued from the study for reasons unrelated to atrial fibrillation.
The company said the safety data for both dosing groups suggests that RSD1235 appeared well-tolerated within the target population.
The company also said there were no cases of drug-related "Torsades de Pointes", a well-characterized arrhythmia which is an occasional side effect of some current anti-arrhythmic drugs.
Karl Keegan, an analyst at Canaccord Adams, said the market overreacted to the news.
So what happened? First of all, atrial fibrillation is the rapid, sometimes uncontrolled beating of the upper chambers of the heart. These rapid beats are related to electrical malfunctions in the heart. [I personally have these all the time from a related syndrome termed WPW]. Most anti-arrhythmic drugs are beta-blockers, which bind to beta adrenergic receptors in the heart and control calcium channel release. RSD1235 works in a similar way but blocks potassium channels in the upper chambers of the heart (atria).
The side effect mentioned of Torsades de Pointes is a drug side effect that affects the ventricles of the heart along with the atria, thus causing ventricular tachycardia which is not good. This tells me that RSD1235 is very specific for its target receptor.
As far as the market overreacting to the news from July trials, how often have we seen that? The drug not meeting efficacy to a significant number happens all the time and should have been factored into the stock price before this.
The study of the investigational drug RSD1235 for the conversion of atrial fibrillation showed that 61 percent of patients in the 300 mg dose completed the study with a normal heart rhythm, compared with 43 percent of all patients receiving a placebo.
Patients in the 600 mg dosing group also showed a 61 percent occurrence of normal heart rhythm compared with 43 percent of patients receiving a placebo, but fell short of expectations on a key comparison.
The data was in line with similar preliminary data Cardiome reported in late July, which sent the stock soaring almost 35 percent in one day after the numbers were released.
Cardiome shares were down C$1.67, or 11.2 percent, at C$13.27 on the Toronto Stock Exchange at midday on a volume of 429,000 shares. On Nasdaq, the shares were off $1.50, or 11.3 percent, at $11.81.
Analysts said investors were looking for improved results above the reported 61 percent in the 600 mg range, from the latest tests.
"This is a continuation of the interim results. I just think that the market got overly enthusiastic with the interim results and we were looking for something much better," said John Maletic, an analyst at Scotia Capital in Toronto.
The Vancouver, British Columbia-based company said 171 patients were successfully cardioverted after the initial three days of dosing and continued in the study, of which 159 either completed the dosing or relapsed to atrial fibrillation.
The remainder of the patients were discontinued from the study for reasons unrelated to atrial fibrillation.
The company said the safety data for both dosing groups suggests that RSD1235 appeared well-tolerated within the target population.
The company also said there were no cases of drug-related "Torsades de Pointes", a well-characterized arrhythmia which is an occasional side effect of some current anti-arrhythmic drugs.
Karl Keegan, an analyst at Canaccord Adams, said the market overreacted to the news.
So what happened? First of all, atrial fibrillation is the rapid, sometimes uncontrolled beating of the upper chambers of the heart. These rapid beats are related to electrical malfunctions in the heart. [I personally have these all the time from a related syndrome termed WPW]. Most anti-arrhythmic drugs are beta-blockers, which bind to beta adrenergic receptors in the heart and control calcium channel release. RSD1235 works in a similar way but blocks potassium channels in the upper chambers of the heart (atria).
The side effect mentioned of Torsades de Pointes is a drug side effect that affects the ventricles of the heart along with the atria, thus causing ventricular tachycardia which is not good. This tells me that RSD1235 is very specific for its target receptor.
As far as the market overreacting to the news from July trials, how often have we seen that? The drug not meeting efficacy to a significant number happens all the time and should have been factored into the stock price before this.
Tuesday, September 12, 2006
Stratagene Highlights Results of FDA's MicroArray Quality Control Project
This is exactly what I was describing in my Term of the Day from September 8th.
Stratagene's Universal Reference RNA Proves Valuable as a Reference Standard for Comparing Results From Many Microarray Platforms
LA JOLLA, Calif.--(Sept. 12, 2006--Stratagene Corporation, a developer, manufacturer and marketer of specialized life science research and diagnostic products, today highlighted the U.S. Food and Drug Administration's (FDA) publication of results from its MicroArray Quality Control (MAQC) project.
As part of the FDA's Critical Path Initiative, the MAQC project aimed to develop standards and quality measures for the microarray community, so that microarrays, as a core technology of pharmacogenomics and toxicogenomics [pharmacology/toxicology at the DNA level of protein expression], could successfully and reliably be used in clinical practice and regulatory decision-making. As a result, the MAQC project will help improve microarray technology and foster its proper applications in the discovery, development and review of FDA regulated products.
"We are proud to have been a part of this collaboration that brought together a broad range of academic, governmental, and commercial organizations, all with the future of microarray technology in mind," said Joseph A. Sorge, MD, President and CEO of Stratagene. "Stratagene's Universal Reference RNA was one of two high-quality reference standards selected as part of the MAQC project. These reference RNAs allow laboratories with many different microarray platforms to compare and share data in the global microarray community."
That is very important for both controls in actually doing the experiments (The RNA needs to be pure and clean) and it's coming from the same source so all the experiments will use the same RNA. NICE!
The project involved six FDA Centers, major providers of microarray platforms and RNA samples, the U.S Environmental Protection Agency, the National Institute of Standards and Technology, academic laboratories, and other stakeholders. By providing the public with large reference datasets along with readily accessible reference RNA samples, the MAQC project aimed to establish quality control metrics and thresholds for objectively assessing the performance achievable by various microarray platforms and evaluating the advantages and disadvantages of various data analysis methods.
For more information about Stratagene's Universal Reference RNAs, visit http://www.stratagene.com/microarrays.
Stratagene is publicly traded on the NASDAQ Index, ticker symbol:STGN
Stratagene's Universal Reference RNA Proves Valuable as a Reference Standard for Comparing Results From Many Microarray Platforms
LA JOLLA, Calif.--(Sept. 12, 2006--Stratagene Corporation, a developer, manufacturer and marketer of specialized life science research and diagnostic products, today highlighted the U.S. Food and Drug Administration's (FDA) publication of results from its MicroArray Quality Control (MAQC) project.
As part of the FDA's Critical Path Initiative, the MAQC project aimed to develop standards and quality measures for the microarray community, so that microarrays, as a core technology of pharmacogenomics and toxicogenomics [pharmacology/toxicology at the DNA level of protein expression], could successfully and reliably be used in clinical practice and regulatory decision-making. As a result, the MAQC project will help improve microarray technology and foster its proper applications in the discovery, development and review of FDA regulated products.
"We are proud to have been a part of this collaboration that brought together a broad range of academic, governmental, and commercial organizations, all with the future of microarray technology in mind," said Joseph A. Sorge, MD, President and CEO of Stratagene. "Stratagene's Universal Reference RNA was one of two high-quality reference standards selected as part of the MAQC project. These reference RNAs allow laboratories with many different microarray platforms to compare and share data in the global microarray community."
That is very important for both controls in actually doing the experiments (The RNA needs to be pure and clean) and it's coming from the same source so all the experiments will use the same RNA. NICE!
The project involved six FDA Centers, major providers of microarray platforms and RNA samples, the U.S Environmental Protection Agency, the National Institute of Standards and Technology, academic laboratories, and other stakeholders. By providing the public with large reference datasets along with readily accessible reference RNA samples, the MAQC project aimed to establish quality control metrics and thresholds for objectively assessing the performance achievable by various microarray platforms and evaluating the advantages and disadvantages of various data analysis methods.
For more information about Stratagene's Universal Reference RNAs, visit http://www.stratagene.com/microarrays.
Stratagene is publicly traded on the NASDAQ Index, ticker symbol:STGN
Labopharm announces Stage III success of Tramadol, Stock Jumps
Labopharm Inc. has announced its analgesic Tramadol, which is in late-stage trials, was found to be a safe alternative for the management of pain.
In a statement, the drugmaker said Tramadol has received regulatory approval in 22 European countries and Mexico and commercial launch of the product across Europe is underway.
For the U.S. market, Labopharm has secured a licensing and distribution agreement with Purdue Pharma.
Tramadol is classified as an mild opiate drug.
Tramadol is a racemic mixture of 2 enantiomers (mirror images of each other), each one displaying differing affinities for various receptors. Tramadol is a selective agonist of mu receptors and preferentially inhibits serotonin reuptake, whereas (-)-tramadol mainly inhibits noradrenaline reuptake. The action of these 2 enantiomers is both complementary and synergistic and results in the analgesic effect. Very interesting mechanism of action.
Labopharm is publically traded under the symbol DDS.TO on the Toronto Exchange.
In a statement, the drugmaker said Tramadol has received regulatory approval in 22 European countries and Mexico and commercial launch of the product across Europe is underway.
For the U.S. market, Labopharm has secured a licensing and distribution agreement with Purdue Pharma.
Tramadol is classified as an mild opiate drug.
Tramadol is a racemic mixture of 2 enantiomers (mirror images of each other), each one displaying differing affinities for various receptors. Tramadol is a selective agonist of mu receptors and preferentially inhibits serotonin reuptake, whereas (-)-tramadol mainly inhibits noradrenaline reuptake. The action of these 2 enantiomers is both complementary and synergistic and results in the analgesic effect. Very interesting mechanism of action.
Labopharm is publically traded under the symbol DDS.TO on the Toronto Exchange.
ALS-357 Phase I/II Initiated by Advanced Life Sciences
ALS-357
Phase I/II Initiated
Therapeutic Area Cancer
Indication Melanoma
Company Name ADVANCED LIFE SCIENCES
Web www.advancedlifesciences.com
Industry: Biotech
Advanced Life Sciences Holdings, Inc., a biopharmaceutical company engaged in the discovery, development and commercialization of novel drugs in the therapeutic areas of infection, cancer and inflammation made an oral presentation and presented a poster at the 232nd American Chemical Society National Meeting. This year's meeting is taking place in San Francisco, California, from September 10 through September 14, 2006. More than 12,500 scientists are expected to attend the Fall meeting. The Company's presentations detailed research on the chemical synthesis and apoptotic activity of caspase-activating pentacyclic triterpenoids.
The caspases belong to a family of protease enzymes that, upon activation, cleave an array of cellular proteins necessary for cell viability. This process of programmed cell death, or apoptosis, is necessary for the removal of unwanted or useless cells. The Company's lead oncology drug candidate, ALS-357, is a pentacyclic triterpenoid with an open IND to initiate Phase I/II clinical trials to treat metastatic melanoma in 2007. ALS-357 has been reported to induce apoptosis in melanoma, leukemia and difficult-to-treat neuroblastoma cell lines. It may target the mitochondria directly in these cancer cells, thus triggering activation of pro-apoptotic proteins involved in internucleosomal DNA fragmentation.
In the ongoing search for molecules effective against cancer with novel mechanisms of action, scientists at Advanced Life Sciences have embarked on the design and chemical synthesis of other novel triterpenoids. New molecules discovered through this research have emerged as potent second generation anti-cancer agents against melanoma, glioblastoma, ovarian and colon cancer cell lines. These molecules, like ALS-357, have been shown to induce apoptosis in these tumor cell lines and may represent potential new anti-cancer drug candidates.
Phase I/II Initiated
Therapeutic Area Cancer
Indication Melanoma
Company Name ADVANCED LIFE SCIENCES
Web www.advancedlifesciences.com
Industry: Biotech
Advanced Life Sciences Holdings, Inc., a biopharmaceutical company engaged in the discovery, development and commercialization of novel drugs in the therapeutic areas of infection, cancer and inflammation made an oral presentation and presented a poster at the 232nd American Chemical Society National Meeting. This year's meeting is taking place in San Francisco, California, from September 10 through September 14, 2006. More than 12,500 scientists are expected to attend the Fall meeting. The Company's presentations detailed research on the chemical synthesis and apoptotic activity of caspase-activating pentacyclic triterpenoids.
The caspases belong to a family of protease enzymes that, upon activation, cleave an array of cellular proteins necessary for cell viability. This process of programmed cell death, or apoptosis, is necessary for the removal of unwanted or useless cells. The Company's lead oncology drug candidate, ALS-357, is a pentacyclic triterpenoid with an open IND to initiate Phase I/II clinical trials to treat metastatic melanoma in 2007. ALS-357 has been reported to induce apoptosis in melanoma, leukemia and difficult-to-treat neuroblastoma cell lines. It may target the mitochondria directly in these cancer cells, thus triggering activation of pro-apoptotic proteins involved in internucleosomal DNA fragmentation.
In the ongoing search for molecules effective against cancer with novel mechanisms of action, scientists at Advanced Life Sciences have embarked on the design and chemical synthesis of other novel triterpenoids. New molecules discovered through this research have emerged as potent second generation anti-cancer agents against melanoma, glioblastoma, ovarian and colon cancer cell lines. These molecules, like ALS-357, have been shown to induce apoptosis in these tumor cell lines and may represent potential new anti-cancer drug candidates.
Term of the Day: Biacore

The BIACORE is an optical biosensor that uses surface plasmon resonance (SPR) for real-time monitoring of macromolecular interactions. (SPR is an light phenomenon that's used to measure changes in solution concentration of molecules). Applications include affinity measurements, binding kinetics, active concentrations, binding specificity and epitope mapping. The technology is for use in drug discovery, antibody screening, ligand fishing and therapeutics. Technical advantages include minimal sample consumption, recovery of surface bound samples for MS analysis, multi-sample analysis and measurement of weak binding events. Typically, protein or DNA is bound by one of several possible methods onto a carboxymethylated dextran-gold surface. The interacting protein of interest is injected over the surface and the kinetics of binding are measured in real time.
Monday, September 11, 2006
Term of the day: Transcription Factor
Transcription factors are proteins that bind directly to their DNA binding sequence to elicit gene expression. Such proteins need some sort activation step, such as phosphorylation/dephosphorylation. A perfect example of activation requiring steps before the protein binds to DNA is nuclear factor Kappa B (NFkB). NFkB is sequestered outside the nucleus by a inhibitor protein termed I kappa B (IkB). IkB is then phosphorylated by IkB kinase, which is then degraded. NFkB then translocates to the nucleus, binds to DNA, and turns on inflammatory genes (genes related to immune function)such as interleukins.
Oscient Shares Rise Despite Safety Issues
© 2006 The Associated Press
WASHINGTON — Shares of Oscient Pharmaceuticals Corp. gained in heavy trading Monday, possibly on speculation that the company could receive positive review for a new use of its drug Factive, despite news that the Food and Drug Administration has safety concerns about the drug.
An FDA panel of experts is scheduled to vote Tuesday on whether the drug, which is currently used to treat certain types of pneumonia and bronchitis, should be used to treat acute sinus infection. More than 30 million adults and children get sinus infections each year, according to the National Institutes of Health. There are concerns the FDA panel might decide not to recommend the sinus infection use for this drug.
According to documents released Monday by the FDA before the panel meeting, patients taking Factive were more likely to develop an allergic rash than those taking other approved products.
JMP Securities analyst Adam Cutler noted that in the briefing documents FDA staff scientists recommended against approving the drug for the new use.
"It's probably a long shot that the committee will recommend approval," Cutler said.
Shares of Waltham, Mass.-based Oscient rose 8 cents, or 7.3 percent, to $1.17 on the Nasdaq in midday trading. In the year to date, the stock has lost nearly half of its value.
Competitor Replidyne Inc. of Louisville, Colo., is also seeking to have the same use approved for its drug Faropenem. Replidyne shares lost 15 cents to $9.90 on the Nasdaq.
Merrill Lynch analyst David Munno said in a note Friday that Faropenem does not carry the same safety concerns as Oscient's drug.
According to Munno, FDA could make a decision on Replidyne's drug by Oct. 20.
Factive is a broad spectrum (different bacteria) antibiotic. It works by inhibiting proteins within the bacteria that regulate it's DNA replication (DNA gyrase and topoisomerase IV). If the bacteria can't replicate, it can't infect you enough to make you sick.
WASHINGTON — Shares of Oscient Pharmaceuticals Corp. gained in heavy trading Monday, possibly on speculation that the company could receive positive review for a new use of its drug Factive, despite news that the Food and Drug Administration has safety concerns about the drug.
An FDA panel of experts is scheduled to vote Tuesday on whether the drug, which is currently used to treat certain types of pneumonia and bronchitis, should be used to treat acute sinus infection. More than 30 million adults and children get sinus infections each year, according to the National Institutes of Health. There are concerns the FDA panel might decide not to recommend the sinus infection use for this drug.
According to documents released Monday by the FDA before the panel meeting, patients taking Factive were more likely to develop an allergic rash than those taking other approved products.
JMP Securities analyst Adam Cutler noted that in the briefing documents FDA staff scientists recommended against approving the drug for the new use.
"It's probably a long shot that the committee will recommend approval," Cutler said.
Shares of Waltham, Mass.-based Oscient rose 8 cents, or 7.3 percent, to $1.17 on the Nasdaq in midday trading. In the year to date, the stock has lost nearly half of its value.
Competitor Replidyne Inc. of Louisville, Colo., is also seeking to have the same use approved for its drug Faropenem. Replidyne shares lost 15 cents to $9.90 on the Nasdaq.
Merrill Lynch analyst David Munno said in a note Friday that Faropenem does not carry the same safety concerns as Oscient's drug.
According to Munno, FDA could make a decision on Replidyne's drug by Oct. 20.
Factive is a broad spectrum (different bacteria) antibiotic. It works by inhibiting proteins within the bacteria that regulate it's DNA replication (DNA gyrase and topoisomerase IV). If the bacteria can't replicate, it can't infect you enough to make you sick.
Cipher Pharma's Tramadol Fails Phase III, Stock down 25%
Cipher Pharmaceuticals (DND.TO) Tramadol Fails Phase 3 Trial; Stock Tumbles 25%
MISSISSAUGA, ON, Sept. 11 /CNW/ - Cipher Pharmaceuticals Inc. (TSX: DND - News) today announced preliminary results from the 02.05 Phase III study of CIP-TRAMADOL ER, an extended-release capsule formulation of the pain medication tramadol. While all three active treatment groups in the study demonstrated a reduction in pain from baseline, the 02.05 efficacy results did not achieve a statistically significant effect relative to placebo with respect to the primary endpoint. A higher than anticipated placebo effect was observed in the control arm. Cipher's New Drug Application (NDA) for CIP-TRAMADOL ER has been accepted for review by the U.S. Food and Drug Administration (FDA) as disclosed by the Company on September 5, 2006. In January 2006, Cipher announced that it had been advised by the FDA that its existing clinical data package met the requirements to file a NDA. The NDA contains data from six completed pharmacokinetic studies and five Phase III studies (three of these providing pivotal efficacy data and two providing long-term safety data). Data analysis on the 02.05 trial is continuing and Cipher will provide the final report on the 02.05 trial to the FDA once it is available.
"The clinical package we submitted to the FDA in June met the requirements for a complete NDA submission as evidenced by the fact it has now been accepted for review. We believe there is sufficient data in the package to support regulatory approval," said Larry Andrews, President and Chief Executive Officer of Cipher Pharmaceuticals. "The 02.05 trial is the latest in a series of six Phase III trials completed by Cipher for CIP-TRAMADOL ER. The high placebo effect observed is disappointing; however, this is not an uncommon occurrence in placebo controlled pain trials. We intend to complete our analysis of the 02.05 study and assess the potential contribution that the higher than anticipated placebo effect may have had on the outcome. This analysis will form part of the final report on the study".
The 02.05 study enrolled 860 patients in a double-blind randomized fixed-dose trial designed to compare efficacy and safety of CIP-TRAMADOL ER with placebo. The primary efficacy endpoint of the study was WOMAC pain intensity. The trial was conducted over a 12-week treatment period in patients with moderate to moderately severe chronic pain from osteoarthritis of the knee or hip. Patients were randomly assigned to one of four arms, a placebo arm and three active arms consisting of a 100 mg, 200 mg, or 300 mg dose of CIP-TRAMADOL ER. Patients in the 100 mg arm were on 100 mg for the entire 12-week period, while patients in the 200 mg and 300 mg arms were titrated up from 100 mg to the respective dosage.
Tramadol is a synthetic opioid that is used to treat moderate to moderately severe pain, which is commonly associated with osteoarthritis, without the severe side-effect profile of morphine and other opioids. Cipher's CIP-TRAMADOL ER capsule is a new formulation that delivers sustained-release drug delivery properties with once-daily dosing. CIP-TRAMADOL ER uses oral controlled-release bead technology developed by Cipher's technology partner, Galephar Pharmaceutical Research
The pain related placebo effect tells you how powerful the mind can be. If you think you are getting better, you will. Amazing.
MISSISSAUGA, ON, Sept. 11 /CNW/ - Cipher Pharmaceuticals Inc. (TSX: DND - News) today announced preliminary results from the 02.05 Phase III study of CIP-TRAMADOL ER, an extended-release capsule formulation of the pain medication tramadol. While all three active treatment groups in the study demonstrated a reduction in pain from baseline, the 02.05 efficacy results did not achieve a statistically significant effect relative to placebo with respect to the primary endpoint. A higher than anticipated placebo effect was observed in the control arm. Cipher's New Drug Application (NDA) for CIP-TRAMADOL ER has been accepted for review by the U.S. Food and Drug Administration (FDA) as disclosed by the Company on September 5, 2006. In January 2006, Cipher announced that it had been advised by the FDA that its existing clinical data package met the requirements to file a NDA. The NDA contains data from six completed pharmacokinetic studies and five Phase III studies (three of these providing pivotal efficacy data and two providing long-term safety data). Data analysis on the 02.05 trial is continuing and Cipher will provide the final report on the 02.05 trial to the FDA once it is available.
"The clinical package we submitted to the FDA in June met the requirements for a complete NDA submission as evidenced by the fact it has now been accepted for review. We believe there is sufficient data in the package to support regulatory approval," said Larry Andrews, President and Chief Executive Officer of Cipher Pharmaceuticals. "The 02.05 trial is the latest in a series of six Phase III trials completed by Cipher for CIP-TRAMADOL ER. The high placebo effect observed is disappointing; however, this is not an uncommon occurrence in placebo controlled pain trials. We intend to complete our analysis of the 02.05 study and assess the potential contribution that the higher than anticipated placebo effect may have had on the outcome. This analysis will form part of the final report on the study".
The 02.05 study enrolled 860 patients in a double-blind randomized fixed-dose trial designed to compare efficacy and safety of CIP-TRAMADOL ER with placebo. The primary efficacy endpoint of the study was WOMAC pain intensity. The trial was conducted over a 12-week treatment period in patients with moderate to moderately severe chronic pain from osteoarthritis of the knee or hip. Patients were randomly assigned to one of four arms, a placebo arm and three active arms consisting of a 100 mg, 200 mg, or 300 mg dose of CIP-TRAMADOL ER. Patients in the 100 mg arm were on 100 mg for the entire 12-week period, while patients in the 200 mg and 300 mg arms were titrated up from 100 mg to the respective dosage.
Tramadol is a synthetic opioid that is used to treat moderate to moderately severe pain, which is commonly associated with osteoarthritis, without the severe side-effect profile of morphine and other opioids. Cipher's CIP-TRAMADOL ER capsule is a new formulation that delivers sustained-release drug delivery properties with once-daily dosing. CIP-TRAMADOL ER uses oral controlled-release bead technology developed by Cipher's technology partner, Galephar Pharmaceutical Research
The pain related placebo effect tells you how powerful the mind can be. If you think you are getting better, you will. Amazing.
Genentech Receives FDA Letter for More Data on Proposed Expanded Use of Avastin
Genentech Receives FDA Letter for More Data on Proposed Expanded Use of Avastin SAN FRANCISCO (AP) -- Drug maker Genentech Inc. said Monday the Food and Drug Administration had sent it a complete response letter asking for more safety and efficacy information on a new application for its metastatic breast cancer treatment Avastin.
The company had asked the FDA to approve Avastin's use as a first-line therapy in conjunction with chemotherapy for metatastic breast cancer. The request means the company will have to recollect information from study sites.
The study was sponsored by the National Cancer Institute and conducted by a network of researchers led by the Eastern Cooperative Oncology Group. Genentech had submitted interim data from the trial in May.
Genentech anticipates resubmitting the data by the middle of 2007. The FDA will start a new six-month review once the data is submitted, the company said.
Avastin was approved in 2004 for the treatment of metastatic colorectal cancer. The company has been testing Avastin as a treatment for several other types of cancer. The company halted a trial testing its effectiveness on pancreatic cancer in June, after early data showed it was not significantly effective.
Shares of Genentech fell $3.16, or 3.9 percent, to $78.91 on the New York Stock Exchange in morning trading. The stock has traded between $75.58 and $100.20 over the last 52 weeks.
Metastatic breast cancer means that the cancer is relocating to other parts of the body. Usually, breast cancer cells migrate to bone and liver making it very hard to treat. Bone metastases are very nasty since radiation cannot fully penetrate bone.
Avastin is an anti-angiogenic drug that stops tumors from making their own networks of blood vessels to supply it. Avastin is a monoclonal antibody directed to bind to vascular endothelial growth factor. Basically, it binds to the signal that tells cells to make new blood vessels. Without an adequate blood supply, a tumor that wants to grow rapidly, needs a big, fresh blood supply. Without that, it shrinks and dies.
Genentech trades under the symbol DNA.
The company had asked the FDA to approve Avastin's use as a first-line therapy in conjunction with chemotherapy for metatastic breast cancer. The request means the company will have to recollect information from study sites.
The study was sponsored by the National Cancer Institute and conducted by a network of researchers led by the Eastern Cooperative Oncology Group. Genentech had submitted interim data from the trial in May.
Genentech anticipates resubmitting the data by the middle of 2007. The FDA will start a new six-month review once the data is submitted, the company said.
Avastin was approved in 2004 for the treatment of metastatic colorectal cancer. The company has been testing Avastin as a treatment for several other types of cancer. The company halted a trial testing its effectiveness on pancreatic cancer in June, after early data showed it was not significantly effective.
Shares of Genentech fell $3.16, or 3.9 percent, to $78.91 on the New York Stock Exchange in morning trading. The stock has traded between $75.58 and $100.20 over the last 52 weeks.
Metastatic breast cancer means that the cancer is relocating to other parts of the body. Usually, breast cancer cells migrate to bone and liver making it very hard to treat. Bone metastases are very nasty since radiation cannot fully penetrate bone.
Avastin is an anti-angiogenic drug that stops tumors from making their own networks of blood vessels to supply it. Avastin is a monoclonal antibody directed to bind to vascular endothelial growth factor. Basically, it binds to the signal that tells cells to make new blood vessels. Without an adequate blood supply, a tumor that wants to grow rapidly, needs a big, fresh blood supply. Without that, it shrinks and dies.
Genentech trades under the symbol DNA.
Sunday, September 10, 2006
FDA Advises Aspirin Users That Ibuprofen Can Limit Drug's Effect
WASHINGTON (AP) -- The Food and Drug Administration is advising patients who take aspirin to prevent heart attacks that taking ibuprofen for pain relief at the same time can diminish aspirin's effectiveness.
According to an advisory released Friday, ibuprofen interferes with the anti-platelet effect that helps aspirin prevent heart attacks and stroke. The agency says patients can minimize the counteraction between the drugs by limiting their use of ibuprofen.
Ibuprofen is marketed under a variety of brands, including Wyeth's Advil and Abbott Laboratories' Brufen. Aspirin is marketed in the United States by Bayer AG.
Calls placed to makers of ibuprofen were not immediately returned.
Shares of Wyeth rose 4 cents Friday to close at $48.25 on the New York Stock Exchange. Abbott's stock rose $1 to close at $48.90, while Bayer AG rose 22 cents to close at $48.37, both on the NYSE.
What does that mean?
Platelets are non-nucleated blood cells that initiate blood clotting. Aspirin has long been used as a blood thinner to treat and prevent stroke and heart related clotting problems. It's primary mechanism of action depletes platelets from the circulation and inhibiting a protein in the clotting pathway (COX, cyclooxygenase). Ibuprofen blocks the binding site to which aspirin binds to, resulting in greater than 90% inhibition of activity.
According to an advisory released Friday, ibuprofen interferes with the anti-platelet effect that helps aspirin prevent heart attacks and stroke. The agency says patients can minimize the counteraction between the drugs by limiting their use of ibuprofen.
Ibuprofen is marketed under a variety of brands, including Wyeth's Advil and Abbott Laboratories' Brufen. Aspirin is marketed in the United States by Bayer AG.
Calls placed to makers of ibuprofen were not immediately returned.
Shares of Wyeth rose 4 cents Friday to close at $48.25 on the New York Stock Exchange. Abbott's stock rose $1 to close at $48.90, while Bayer AG rose 22 cents to close at $48.37, both on the NYSE.
What does that mean?
Platelets are non-nucleated blood cells that initiate blood clotting. Aspirin has long been used as a blood thinner to treat and prevent stroke and heart related clotting problems. It's primary mechanism of action depletes platelets from the circulation and inhibiting a protein in the clotting pathway (COX, cyclooxygenase). Ibuprofen blocks the binding site to which aspirin binds to, resulting in greater than 90% inhibition of activity.
Subscribe to:
Posts (Atom)