Companies often refer to their products in their pipelines as "drugs targeted to disrupt oncogene function". So what are oncogenes? Simply, they are DNA encoded genes that have been proven experimentally to result in tumor formation. To do this, scientists have specialized mice that are "nude" which means they have been manipulated genetically to have no immune function. Then, either the DNA gene is put into the animal and made to express it's coded protein or cells already expressing the gene are injected under the skin of the animal. If a tumor develops, that protein is oncogenic. Usually, oncogenes are normal proteins within the body, but because of mutations or ?, they are either over-expressed (too much protein) or absent/non-functional.
(BTW- nude mice are called nude because they have no fur. When their immune function is suppressed genetically, a marker gene was inserted such that the scientist knows for sure that the animal has no immune system because it has no fur.)
Examples of oncogenes are Ras (always activated), Her2 (overexpressed), p53 (deletion), BCR/ABL ( a fusion of two proteins).
All of the above examples are active targets in Biotech/Pharma product pipelines.
Sunday, September 10, 2006
Barr (BRL :  BRL54.08, +0.55, +1.0% ) slid 3% to close at $53.53 on word that the Food and Drug Administration had granted Watson (WPI :
BRL54.08, +0.55, +1.0% ) slid 3% to close at $53.53 on word that the Food and Drug Administration had granted Watson (WPI : ![]() WPI26.02, +0.29, +1.1% ) final approval to put out a generic version of Barr's Seasonale oral contraceptive. Watson, which plans to market the product under the name Quasense, said the contraceptive had sales of $110 million for the twelve months ended June 2006.
WPI26.02, +0.29, +1.1% ) final approval to put out a generic version of Barr's Seasonale oral contraceptive. Watson, which plans to market the product under the name Quasense, said the contraceptive had sales of $110 million for the twelve months ended June 2006.
Barr Laboratories, Inc.
Last: 54.08+0.55+1.03%
4:02pm 09/08/2006
Delayed quote data
4:02pm 09/08/2006
Delayed quote data
Sponsored by:
Watson Pharmaceuticals Inc
Last: 26.02+0.29+1.13%
4:01pm 09/08/2006
Delayed quote data
4:01pm 09/08/2006
Delayed quote data
Sponsored by:
Watson closed nominally higher at $25.73.
This is interesting. I am following this as far as the science behind the drug and the legal issues. More to come once the US Market opens on Monday. I'll post the pharmacology ASAP.
This is interesting. I am following this as far as the science behind the drug and the legal issues. More to come once the US Market opens on Monday. I'll post the pharmacology ASAP.
Saturday, September 09, 2006
Term of the day: Tyrosine Kinase
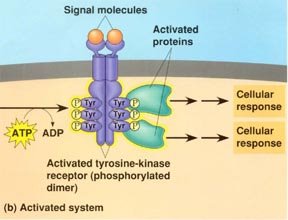
There are a lot of anti-breast cancer therapies now using monoclonal antibodies. In particular, the Epidermal Growth factor family of receptors such as Her2, Her4 and EGFR.
What are these things anyway?
They are classified as receptor tyrosine kinases. Epidermal growth factor, (a soluble peptide secreted from salivary glands, that tells cells to grow) is the ligand for the EGFR. Tyrosine kinases transfer a phosphate molecule to the amino acid tyrosine (Y). This results in 2 major changes in the protein that receives the phosphate group. 1) the protein now has a negative charge attached which 2) causes a conformational change in the structure of the protein.
New York Stock Exchange and Nasdaq companies that have monoclonal antibodies and/or pharmacological inhibitors to the EGFR family are Genentech (DNA), Amgen (AMGN), GlaxoSmithKline (GSK) and AstraZeneca (AZN).
Friday, September 08, 2006
Word of the Day: Microarray
I think this is a good way to learn basic scientific fundamentals/techniques if you are not familiar with the field.
MICROARRAY
An experimental technique that utilizes known control DNA versus unknown (usually tumor derived) DNA samples in order to 1)determine levels of gene expression (abundance) 2)the sequence of the gene. Short sequences of sample DNA are labeled with a fluorescent dye and allowed to bind to gene sequences that are bound to a glass slide or "gene-chip". The non binding DNA's are washed off and the slide is "read" by computer to determine levels of fluorescence or the DNA sequence. The more intense the fluorescence, the gene is highly expressed.
Examples of companies that make the chips (which are very expensive) are Affymetrix (AFFX)and Agilent Tech (A).
MICROARRAY
An experimental technique that utilizes known control DNA versus unknown (usually tumor derived) DNA samples in order to 1)determine levels of gene expression (abundance) 2)the sequence of the gene. Short sequences of sample DNA are labeled with a fluorescent dye and allowed to bind to gene sequences that are bound to a glass slide or "gene-chip". The non binding DNA's are washed off and the slide is "read" by computer to determine levels of fluorescence or the DNA sequence. The more intense the fluorescence, the gene is highly expressed.
Examples of companies that make the chips (which are very expensive) are Affymetrix (AFFX)and Agilent Tech (A).
Dynavax/AstraZeneca Form Alliance to Develop TLR Drugs
BERKELEY, Calif., Sept. 6 /PRNewswire-FirstCall/ -- Dynavax Technologies Corporation (Nasdaq: DVAX - News) has entered into a research collaboration and license agreement with AstraZeneca for the discovery and development of TLR-9 agonist-based therapies for the treatment of asthma and chronic obstructive pulmonary disease (COPD). The collaboration will utilize Dynavax's proprietary second-generation TLR-9 agonist immunostimulatory sequences or ISS.
Under the terms of the agreement, Dynavax and AstraZeneca will collaborate to identify lead TLR-9 agonists and conduct appropriate research phase studies. AstraZeneca will be responsible for any development and worldwide commercialization of products arising out of the research program. Dynavax may also have the opportunity to co-promote in the United States products arising from the collaboration.
Financial terms of the collaboration include an upfront fee of $10 million plus research funding and preclinical milestones that could bring the total committed funding to $27 million, resulting in a total potential deal value of approximately $136 million. Upon commercialization, Dynavax is also eligible to receive royalties based on product sales.
"The management of respiratory diseases such as asthma and COPD remains a major health challenge affecting 150 million people worldwide and representing a significant financial burden to the global healthcare system," said Claude Bertrand, Vice President Respiratory and Inflammation Research at AstraZeneca. "New approaches that have the potential to reverse the course of respiratory disease are needed. AstraZeneca believes that Dynavax's ISS-based technology represents an innovative, next-generation therapeutic intervention that could potentially expand and strengthen AstraZeneca's strong position in the respiratory disease field."
"We believe that AstraZeneca is the ideal partner for the development of asthma and COPD ISS-based therapies, as they have one of the most widely respected and commercially successful respiratory product portfolios in the industry," said Dino Dina, MD, Dynavax's chief executive officer. "We appreciate AstraZeneca's recognition of the innovative, disease-modifying potential of our TLR-9 agonist based approaches and are optimistic that with our combined resources and know-how, we will be able to create novel therapeutics that may provide benefit to patients suffering from these diseases."
Continued Dr. Dina: "The clinical data generated by our four Phase 2 clinical trials for the treatment of ragweed-induced allergic rhinitis provide a strong foundation for applying ISS-based agonists to treat asthma and COPD. We are hopeful that this collaboration, the goal of which is to explore the potential of ISS alone to treat respiratory diseases, will help to expand our existing portfolio of ISS-based products."
So What does this mean? What are "TLRs" Anyway! --->About TLR-9 TLR is an acronym for Toll Like Receptor. Toll receptors were first characterized in the fly (Drosophilia) and more recently a related family of Toll like receptors in the vertebrate genome. They play a central role in innate immunity and are required for the adaptive immune response. [innate immunity is non-cell mediated, adaptive is mediated by cells of the immune system.] These receptors respond and recognize molecules from bacterial, viral and fungal pathogens. For example; if you eat bacterial contaminated food, the TLR receptors in your gut will recognize specific parts of the bacterial cell wall and essentially "yell for help". Other cells, such as natural killer and dendritic, then bolster the immune response by cytokine release.
Under the terms of the agreement, Dynavax and AstraZeneca will collaborate to identify lead TLR-9 agonists and conduct appropriate research phase studies. AstraZeneca will be responsible for any development and worldwide commercialization of products arising out of the research program. Dynavax may also have the opportunity to co-promote in the United States products arising from the collaboration.
Financial terms of the collaboration include an upfront fee of $10 million plus research funding and preclinical milestones that could bring the total committed funding to $27 million, resulting in a total potential deal value of approximately $136 million. Upon commercialization, Dynavax is also eligible to receive royalties based on product sales.
"The management of respiratory diseases such as asthma and COPD remains a major health challenge affecting 150 million people worldwide and representing a significant financial burden to the global healthcare system," said Claude Bertrand, Vice President Respiratory and Inflammation Research at AstraZeneca. "New approaches that have the potential to reverse the course of respiratory disease are needed. AstraZeneca believes that Dynavax's ISS-based technology represents an innovative, next-generation therapeutic intervention that could potentially expand and strengthen AstraZeneca's strong position in the respiratory disease field."
"We believe that AstraZeneca is the ideal partner for the development of asthma and COPD ISS-based therapies, as they have one of the most widely respected and commercially successful respiratory product portfolios in the industry," said Dino Dina, MD, Dynavax's chief executive officer. "We appreciate AstraZeneca's recognition of the innovative, disease-modifying potential of our TLR-9 agonist based approaches and are optimistic that with our combined resources and know-how, we will be able to create novel therapeutics that may provide benefit to patients suffering from these diseases."
Continued Dr. Dina: "The clinical data generated by our four Phase 2 clinical trials for the treatment of ragweed-induced allergic rhinitis provide a strong foundation for applying ISS-based agonists to treat asthma and COPD. We are hopeful that this collaboration, the goal of which is to explore the potential of ISS alone to treat respiratory diseases, will help to expand our existing portfolio of ISS-based products."
So What does this mean? What are "TLRs" Anyway! --->About TLR-9 TLR is an acronym for Toll Like Receptor. Toll receptors were first characterized in the fly (Drosophilia) and more recently a related family of Toll like receptors in the vertebrate genome. They play a central role in innate immunity and are required for the adaptive immune response. [innate immunity is non-cell mediated, adaptive is mediated by cells of the immune system.] These receptors respond and recognize molecules from bacterial, viral and fungal pathogens. For example; if you eat bacterial contaminated food, the TLR receptors in your gut will recognize specific parts of the bacterial cell wall and essentially "yell for help". Other cells, such as natural killer and dendritic, then bolster the immune response by cytokine release.
Asian Markets Up on Friday Trading..
Could be a good sign for us when US Markets open. Biotech needs some good news before the weekend. More later today....
Thursday, September 07, 2006
Tumor Survey--Cancer Genes Cataloged.
Good background info for cancer research.
Tumour survey unearths wealth of mutants
Cataloguing cancer genes may pay dividends.
Helen Pearson
A hunt through thousands of human genes has turned up nearly 200 that are altered in breast and colon cancer. These genes might be useful for diagnosing cancer or as new targets for drugs.
The findings suggest that a US$1.5-billion initiative funded by the US government to decode the 'cancer genome' could throw up useful leads — and goes some way towards appeasing the project's critics.
Cancer arises when cells rack up mutations in a number of their genes, and begin to divide uncontrollably. Researchers have already identified some of the genes involved in this process by cherry-picking promising candidates — but there are thought to be many, many more.
In the latest study, researchers searched for culprits by determining the genetic sequence of some 13,000 genes found in 11 breast tumours and 11 colorectal tumours that had been preserved for study. They then looked for differences in the genes between cancerous and normal tissues, and cross-checked the result with an additional bank of tumours from 24 breast or colorectal cancers.
The trawl unearthed a total of 189 genes that were mutated in the tumours and are suspected to be a cause of cancer; the majority of these had not been implicated in cancer before.
Fickle enemy
The study confirms that cancer is a fiendishly fickle enemy. The team found that breast and colon tumours harbour almost completely different mutations — in fact, only two mutated genes were shared between them. Cancers in other tissues might also be driven by a different spectrum of mutations.
In addition, the team found that no two tumours are exactly alike. All in all, the researchers estimate that a typical breast tumour carries mutations in more than 100 genes. Some 20 of these might be involved in causing the cancer, they say. Less than half of these are likely to be found in another breast tumour. The study is published in Science1.
SOURCE- Nature.com
Tumour survey unearths wealth of mutants
Cataloguing cancer genes may pay dividends.
Helen Pearson
A hunt through thousands of human genes has turned up nearly 200 that are altered in breast and colon cancer. These genes might be useful for diagnosing cancer or as new targets for drugs.
The findings suggest that a US$1.5-billion initiative funded by the US government to decode the 'cancer genome' could throw up useful leads — and goes some way towards appeasing the project's critics.
Cancer arises when cells rack up mutations in a number of their genes, and begin to divide uncontrollably. Researchers have already identified some of the genes involved in this process by cherry-picking promising candidates — but there are thought to be many, many more.
In the latest study, researchers searched for culprits by determining the genetic sequence of some 13,000 genes found in 11 breast tumours and 11 colorectal tumours that had been preserved for study. They then looked for differences in the genes between cancerous and normal tissues, and cross-checked the result with an additional bank of tumours from 24 breast or colorectal cancers.
The trawl unearthed a total of 189 genes that were mutated in the tumours and are suspected to be a cause of cancer; the majority of these had not been implicated in cancer before.
Fickle enemy
The study confirms that cancer is a fiendishly fickle enemy. The team found that breast and colon tumours harbour almost completely different mutations — in fact, only two mutated genes were shared between them. Cancers in other tissues might also be driven by a different spectrum of mutations.
In addition, the team found that no two tumours are exactly alike. All in all, the researchers estimate that a typical breast tumour carries mutations in more than 100 genes. Some 20 of these might be involved in causing the cancer, they say. Less than half of these are likely to be found in another breast tumour. The study is published in Science1.
SOURCE- Nature.com
Genomic Health, Inc. Stock up 18% today
I noticed this while looking at today's most actives. Interesting company profile in that they are investigating cancer at the genomic (DNA) level.They use this information for diagnostic use for individual patients as well. One pipeline diagnostic kit looks at Estrogen positive tumors vs. negative tumors in breast cancers for example. The same is true in men with prostate cancer, which reverts to androgen independent as the disease progresses. Such diagnostic kits would be invaluable to the physician in determining courses of treatment. Pretty cool.
Wednesday, September 06, 2006
An Update on Genta, Inc.
NEW YORK (AP) -- Drug developer Genta Inc. said Wednesday a Food and Drug Administration advisory committee recommended against approving the company's leukemia treatment candidate. The panel's recommendation is part of an ongoing review by the FDA for the drug candidate Genasense, an injectable drug aimed at treating a form of blood cancer called refractory chronic lymphocytic leukemia, in conjunction with chemotherapy. The FDA is expected to decide on whether to approve the drug by Oct. 29 and usually follows the recommendations of its advisory committees.
This drug is most likely dead in the water. Ouch.
This drug is most likely dead in the water. Ouch.
Asian Markets down in thursday trading...
"We're running out of good news to push up the market," said Jackson Wong, investment manager at Tanrich Securities in Hong Kong. "Worries about inflation in the U.S. are coming back, and that is hurting tech stocks."
More to come today once the US Market opens. Can biotech continue to get pounded?
More to come today once the US Market opens. Can biotech continue to get pounded?
Tuesday, September 05, 2006
A Brief Review of Clinical Trials: What do the Different Phases Mean?
It may be difficult to interpret the data depending on what stage of development a particular compound resides, such as Phase IIb versus Phase IV. So let's be concrete on what each stage of clinical development REALLY means from a pharmacology point of view with a summation question at the end:
Ok, once a drug is judged ready to be studied in humans, a Notice of Claimed Investigational Exemption for a new drug (IND) must be filed with the FDA. The IND includes (1) information on the composition and source of the drug, (2) manufacturing info, (3) all data from animal studies, (4) clinical plans and protocols, and (5) the names and credentials of the physicians who will conduct the trials. Once submitted,
PHASE 1. The effects of the drug as a function of dosage are established in a small number of healthy volunteers. This is done to determine whether humans and animals show significantly different responses to the drug and to establish the probable limits of the safe clinical dose range. The trial is "open" which means both investigators and subjects know what is being given. Basically two questions are being asked here: 1- Is it safe and 2- what are the pharmacokinetics?
PHASE 2. The drug is studied for the first time in patients with the target disease to determine its efficacy. Small numbers are studied in GREAT detail. Usually performed in special clinical centers such as University Hospitals. QUESTION- Does it work in patients?
PHASE 3. The drug is evaluated in much larger numbers of patients. Many times this number is in the thousands, to further establish efficacy and safety. These trials are designed to minimize the errors caused by placebo effects, variable course of disease, and so on. Phase 3 are usually performed in settings similar to those anticipated for the ultimate use of the drug. If phase 3 results meet expectations, application will be made for permission to market the new agent. QUESTION- Does it work double blind?
PHASE 4. Monitoring of the new drug under actual conditions of use in large numbers of patients. Physicians report any adverse side effects over long term dosing in large, large patient numbers. Any side effects are noted and submitted to the FDA. SUMMARY- All perscribed clinical drugs are essentially in this phase. They are monitored for long term use effects, if any.
Ok, once a drug is judged ready to be studied in humans, a Notice of Claimed Investigational Exemption for a new drug (IND) must be filed with the FDA. The IND includes (1) information on the composition and source of the drug, (2) manufacturing info, (3) all data from animal studies, (4) clinical plans and protocols, and (5) the names and credentials of the physicians who will conduct the trials. Once submitted,
PHASE 1. The effects of the drug as a function of dosage are established in a small number of healthy volunteers. This is done to determine whether humans and animals show significantly different responses to the drug and to establish the probable limits of the safe clinical dose range. The trial is "open" which means both investigators and subjects know what is being given. Basically two questions are being asked here: 1- Is it safe and 2- what are the pharmacokinetics?
PHASE 2. The drug is studied for the first time in patients with the target disease to determine its efficacy. Small numbers are studied in GREAT detail. Usually performed in special clinical centers such as University Hospitals. QUESTION- Does it work in patients?
PHASE 3. The drug is evaluated in much larger numbers of patients. Many times this number is in the thousands, to further establish efficacy and safety. These trials are designed to minimize the errors caused by placebo effects, variable course of disease, and so on. Phase 3 are usually performed in settings similar to those anticipated for the ultimate use of the drug. If phase 3 results meet expectations, application will be made for permission to market the new agent. QUESTION- Does it work double blind?
PHASE 4. Monitoring of the new drug under actual conditions of use in large numbers of patients. Physicians report any adverse side effects over long term dosing in large, large patient numbers. Any side effects are noted and submitted to the FDA. SUMMARY- All perscribed clinical drugs are essentially in this phase. They are monitored for long term use effects, if any.
Shares of drug developer Genta Inc. dropped sharply Tuesday after the Food and Drug Administration questioned whether its leukemia treatment candidate would offer any substantial improvement over chemotherapy.
In a summary prepared for an advisory panel that meets Wednesday, the FDA staff said 10 percent more patients with chronic lymphocytic leukemia (CLL) responded to chemotherapy when Genasense was added, but the difference "is of questionable clinical significance." Adding infusions of Genasense also "appears to add to the toxicity of the treatment," the FDA staff said. Genta's figures showed more patients experienced nausea, fever, fatigue, headaches and other problems.
The response rate, or complete response to the treatment, was 17 percent, compared with a 7 percent response rate for chemotherapy alone. That falls short of a 20 percent rate of remission goal. Also, the median survival rate for patients in the 223-person study on a combination of Genasense and chemotherapy was 33.8 months. The group solely on chemotherapy had a median survival rate of 32.9 months.
THIS is a common theme in analyzing clinical data. Although the drug might be synergistic with chemotherapy in raising the survival rate by a year, overall, when the patient numbers are very large there is no difference. BTW, refractive CLL is a nasty disease and not as treatable as primary ALL and NHL.
In a summary prepared for an advisory panel that meets Wednesday, the FDA staff said 10 percent more patients with chronic lymphocytic leukemia (CLL) responded to chemotherapy when Genasense was added, but the difference "is of questionable clinical significance." Adding infusions of Genasense also "appears to add to the toxicity of the treatment," the FDA staff said. Genta's figures showed more patients experienced nausea, fever, fatigue, headaches and other problems.
The response rate, or complete response to the treatment, was 17 percent, compared with a 7 percent response rate for chemotherapy alone. That falls short of a 20 percent rate of remission goal. Also, the median survival rate for patients in the 223-person study on a combination of Genasense and chemotherapy was 33.8 months. The group solely on chemotherapy had a median survival rate of 32.9 months.
THIS is a common theme in analyzing clinical data. Although the drug might be synergistic with chemotherapy in raising the survival rate by a year, overall, when the patient numbers are very large there is no difference. BTW, refractive CLL is a nasty disease and not as treatable as primary ALL and NHL.
US Market News 9-05-06
Adolor shares down 46% on drug's study results
Tuesday September 5, 12:06 pm ET
Adolor and GlaxoSmithKline reported mixed results from the latest clinical tests for Entereg, an experimental treatment for bowel disorders suffered by patients taking opioids for pain relief.
The Food and Drug Administration issued an approvable letter for Entereg last year, but the agency requested additional clinical-trial data before it would issue final approval.
On Tuesday, the companies said Entereg achieved statistical significance in its primary goal of helping patients achieve three or more spontaneous bowel movements in one of two studies.
Adolor was trading down 46 percent at $13.42 Tuesday. GlaxoSmithKline was down 1 percent at $56.21.
Entereg was develop by Adolor, which in 2002 entered into a co-development and promotion agreement with GlaxoSmithKline for it.
Published September 5, 2006 by the Philadelphia Business Journal
So, What happened here? What does this drug do? While one of two identical phase III studies achieved its goal, the other did not. A phase IIb study of the drug also failed to reach its goal. However, both of the trials that missed their main targets did achieve a secondary objective of altering the average weekly frequency of spontaneous bowel movements. Entereg was developed to relieve non cancerous pain in the bowel caused by opioid abuse. Of course, the most commonly abused opioids are heroin, morphine and oxycodone. Constipation has long been recognized as an effect of opioids. Opioid receptors (G proteins) exist in high density within the gastrointestinal tract, and the constipating effects are mediated through the GI nerve system. Also, the stomach action of pushing food through the small intestine is decreased as well as stomach acid production. Peristaltic waves that move intestinal contents are also diminished (think of how a caterpillar crawls in waves, same thing). So its clear why they need a drug like Entereg, but pulling it out of phase III most likely means it did not meet efficacy in a large number of patients.
Tuesday September 5, 12:06 pm ET
Adolor and GlaxoSmithKline reported mixed results from the latest clinical tests for Entereg, an experimental treatment for bowel disorders suffered by patients taking opioids for pain relief.
The Food and Drug Administration issued an approvable letter for Entereg last year, but the agency requested additional clinical-trial data before it would issue final approval.
On Tuesday, the companies said Entereg achieved statistical significance in its primary goal of helping patients achieve three or more spontaneous bowel movements in one of two studies.
Adolor was trading down 46 percent at $13.42 Tuesday. GlaxoSmithKline was down 1 percent at $56.21.
Entereg was develop by Adolor, which in 2002 entered into a co-development and promotion agreement with GlaxoSmithKline for it.
Published September 5, 2006 by the Philadelphia Business Journal
So, What happened here? What does this drug do? While one of two identical phase III studies achieved its goal, the other did not. A phase IIb study of the drug also failed to reach its goal. However, both of the trials that missed their main targets did achieve a secondary objective of altering the average weekly frequency of spontaneous bowel movements. Entereg was developed to relieve non cancerous pain in the bowel caused by opioid abuse. Of course, the most commonly abused opioids are heroin, morphine and oxycodone. Constipation has long been recognized as an effect of opioids. Opioid receptors (G proteins) exist in high density within the gastrointestinal tract, and the constipating effects are mediated through the GI nerve system. Also, the stomach action of pushing food through the small intestine is decreased as well as stomach acid production. Peristaltic waves that move intestinal contents are also diminished (think of how a caterpillar crawls in waves, same thing). So its clear why they need a drug like Entereg, but pulling it out of phase III most likely means it did not meet efficacy in a large number of patients.
Monday, September 04, 2006
Mission Statement to the forum....
My vision for this forum is to interpret and explain the scientific aspects of biotech/pharma in relation to investing into a particular company. For example, when company "X" releases clinical trial data and the stock does nothing you may ask yourself why? You then read the trial data and something like "the internalization of the Erb4 receptor was not statistically significant" however "the dual dimerization with EGFR was inhibited thus resulting in decreased cellular differentiation". What does this mean? Basically, companies use these large scientific words to say what could be summed up in one sentence: The main effect of drug X did not work for what we were looking for but there are signs of drug action so we will need to continue the trial for additional data.
I look forward to interacting with those of you interested in understanding biotech/pharma.
I look forward to interacting with those of you interested in understanding biotech/pharma.
Subscribe to:
Posts (Atom)

